Decreased IL-10 accelerates B-cell leukemia/lymphoma in a mouse model of pediatric lymphoid leukemia
- PMID: 34727170
- PMCID: PMC8945291
- DOI: 10.1182/bloodadvances.2021005522
Decreased IL-10 accelerates B-cell leukemia/lymphoma in a mouse model of pediatric lymphoid leukemia
Abstract
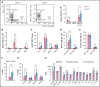
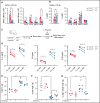
Exposures to a wide repertoire of common childhood infections and strong inflammatory responses to those infections are associated with the risk of pediatric B-cell acute lymphoblastic leukemia (B-ALL) in opposing directions. Neonatal inflammatory markers are also related to risk by unknown mechanism(s). Here, we demonstrate that interleukin-10 (IL-10) deficiency, which is associated with childhood B-ALL, indirectly impairs B lymphopoiesis and increases B-cell DNA damage in association with a module of 6 proinflammatory/myeloid-associated cytokines (IL-1α, IL-6, IL-12p40, IL-13, macrophage inflammatory protein-1β/CCL4, and granulocyte colony-stimulating factor). Importantly, antibiotics attenuated inflammation and B-cell defects in preleukemic Cdkn2a-/-Il10-/- mice. In an ETV6-RUNX1+ (E6R1+) Cdkn2a-/- mouse model of B-ALL, decreased levels of IL-10 accelerated B-cell neoplasms in a dose-dependent manner and altered the mutational profile of these neoplasms. Our results illuminate a mechanism through which a low level of IL-10 can create a risk for leukemic transformation and support developing evidence that microbial dysbiosis contributes to pediatric B-ALL.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



References
-
- Hjalgrim LL, Rostgaard K, Hjalgrim H, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst. 2004;96(20):1549-1556. - PubMed
-
- Chang JS, Tsai C-R, Tsai Y-W, Wiemels JL. Medically diagnosed infections and risk of childhood leukaemia: a population-based case-control study. Int J Epidemiol. 2012;41(4):1050-1059. - PubMed

