Combining selective serotonin reuptake inhibitors and cognitive behavioral therapy in youth with depression and anxiety
- PMID: 34728290
- PMCID: PMC8674898
- DOI: 10.1016/j.jad.2021.10.047
Combining selective serotonin reuptake inhibitors and cognitive behavioral therapy in youth with depression and anxiety
Abstract
Background: Treatment studies of children and adolescents with internalizing disorders suggest that the combination of a selective serotonin reuptake inhibitor (SSRI) and cognitive behavioral therapy (CBT) consistently produces greater improvement than either treatment alone. We sought to determine how response to combined treatment varies across disorders (anxiety versus depression), and by specific patient characteristics.
Methods: Three large National Institutes of Health-funded trials of children and adolescents with major depression (n = 2) and anxiety disorders (n = 1) were evaluated, each comparing CBT + SSRI to SSRI only, Bayesian Hierarchical Models (BHMs) were used, for endpoint response, time course of response and predictors of response in participants who received SSRI or SSRI+CBT.
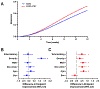
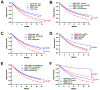
Results: SSRI+CBT significantly decreased symptoms by week 4 (p<0.001) across disorders. This improvement continued at week 8 and 12 (p<0.001); however, the additive benefit of CBT over SSRI monotherapy was not statistically significant until week 12 (p<0.001). The fastest response to SSRI+CBT was for patients who were younger, with milder baseline anxiety/depression symptoms and depressive disorders. The slowest response for SSRI+CBT was for boys, adolescents, minoritized children, those with severe symptoms and externalizing disorders.
Limitations: Limitations included inconsistent moderators, variation in the number of observations over time and a lack of genetic or pharmacokinetic variables related to SSRI exposure across studies.
Conclusions: The superiority of SSRI+CBT for youth with depression and anxiety is further supported. For purposes of rapid and greater relief, combination treatment is the superior approach across anxiety and depression and is robust to a range of participant characteristics. However, the added value of CBT (with an SSRI) occurs late in treatment. These findings represent a step towards understanding heterogeneity of treatment response and raise the possibility that interventions could be better tailored or adapted based on patient characteristics.
Keywords: Anxiety; Clinical trial; Depression; Fluoxetine; Major depressive disorder; Paroxetine; SSRI; Sertraline.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures

References
-
- Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, Iyengar S, Shamseddeen W, Ritz L, Mccracken J, Strober M, Suddath R, Leonard H, Porta G, Keller M, Brent D, 2009. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J. Am. Acad. Child Adolesc. Psychiatry 48, 330–9. doi:10.1097/CHI.0b013e3181977476 - DOI - PMC - PubMed
-
- Bezanson J, Edelman A, Karpinski S, Shah VB, 2014. Julia: A Fresh Approach to Numerical Computing 59, 65–98.
-
- Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan ND, Zelazny J, Onorato M, Kennard B, Mayes TL, DeBar LL, McCracken JT, Strober M, Suddath R, Leonard H, Porta G, Keller MB, 2009. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am. J. Psychiatry 166, 418–426. doi:10.1176/appi.ajp.2008.08070976 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

