Ultra-long-acting (XLA) antivirals for chronic viral hepatitis
- PMID: 34728344
- PMCID: PMC9204217
- DOI: 10.1016/j.ijid.2021.10.052
Ultra-long-acting (XLA) antivirals for chronic viral hepatitis
Abstract
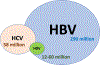
Viral hepatitis is among the top four causes of mortality globally, causing 1.4 million deaths each year, exceeding tuberculosis, malaria and human immunodeficiency virus. Hepatitis B and C are responsible for 90% of hepatitis deaths, and the remaining 10% are caused by other hepatitis viruses. The annual number of deaths from hepatitis C is declining, whereas the numbers of deaths from hepatitis B and D are increasing. Hepatitis B alone represents the seven highest cause of mortality worldwide. Spurred on by development of curative antivirals for hepatitis C and expanding access to hepatitis B virus (HBV) vaccination, the World Health Organization has committed to eliminating viral hepatitis as a public health threat by 2030. Like the majority of current antivirals, those available for HBV are virostatic. They are capable of suppressing viral replication but cannot eliminate the virus from infected patients. Therefore, treatment is lifelong. Long-term adherence to medication continues to represent a major challenge. Importantly, HBV often reactivates, leading to potential life-threatening events in immunosuppressed patients. Therapeutic options are limited for hepatitis D; however, promising new, effective antivirals are on the horizon. Recent advances have emerged in medicinal chemistry and drug delivery approaches to produce ultra-long-acting (XLA) antivirals. These can extend antiviral activity from months to 1 year or even longer. These new formulations can overcome the challenges of daily dosing and maximize drug exposure. The development of XLA antivirals targeting viral hepatitis may also facilitate cure strategies.
Keywords: Chemical vaccines; Hepatitis B; Hepatitis C; Hepatitis D; Long-acting antivirals; Prevention; Viral hepatitis.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement BE and HG hold stocks in EXAVIR, a biotech company. The other authors declare no financial conflict of interests.
Figures



References
-
- Alfaiate D, Clément S, Gomes D, Goossens N, Negro F. Chronic hepatitis D and hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. J Hepatol 2020;73:533–9. - PubMed
-
- Asselah T, Loureiro D, Le Gal F, Narguet S, Brichler S, Bouton V, et al.. Early virolog-ical response in six patients with hepatitis D virus infection and compensated cirrhosis treated with bulevirtide in real-life. Liver Int 2021;41:1509–17. - PubMed
-
- Benitez-Gutierrez L, Soriano V, Requena S, Arias A, Barreiro PO, de Mendoza C. Treatment and prevention of HIV infection with long-acting antiretrovirals. Expert Rev Clin Pharmacol 2018;11:507–17. - PubMed
-
- Cooke G, Andrieux-Meyer I, Applegate T, Atun R, Burry J, Cheinquer H, et al.. Accelerating the elimination of viral hepatitis. Lancet Gastroenterol Hepatol 2019;4:135–84. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 NS097195/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- R01 AI158160/AI/NIAID NIH HHS/United States
- T32 NS105594/NS/NINDS NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- R01 MH121402/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- T32 NS007488/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- R01 DA054535/DA/NIDA NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS126089/NS/NINDS NIH HHS/United States
- R13 NS083315/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

