Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy
- PMID: 34728497
- PMCID: PMC8564865
- DOI: 10.1212/NXI.0000000000001098
Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy
Erratum in
-
Clinical and Laboratory Features in Anti-NF155 Autoimmune Nodopathy.Neurol Neuroimmunol Neuroinflamm. 2021 Dec 20;9(1):e1129. doi: 10.1212/NXI.0000000000001129. Print 2022 Jan. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 34930829 Free PMC article. No abstract available.
Abstract
Background and objectives: To study the clinical and laboratory features of antineurofascin-155 (NF155)-positive autoimmune nodopathy (AN).
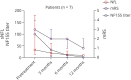
Methods: Patients with anti-NF155 antibodies detected on routine immunologic testing were included. Clinical characteristics, treatment response, and functional scales (modified Rankin Scale [mRS] and Inflammatory Rasch-built Overall Disability Scale [I-RODS]) were retrospectively collected at baseline and at the follow-up. Autoantibody and neurofilament light (NfL) chain levels were analyzed at baseline and at the follow-up.
Results: Forty NF155+ patients with AN were included. Mean age at onset was 42.4 years. Patients presented with a progressive (75%), sensory motor (87.5%), and symmetric distal-predominant weakness in upper (97.2%) and lower extremities (94.5%), with tremor and ataxia (75%). Patients received a median of 3 (2-4) different treatments in 46 months of median follow-up. Response to IV immunoglobulin (86.8%) or steroids (72.2%) was poor in most patients, whereas 77.3% responded to rituximab. HLA-DRB1*15 was detected in 91.3% of patients. IgG4 anti-NF155 antibodies were predominant in all patients; anti-NF155 titers correlated with mRS within the same patient (r = 0.41, p = 0.004). Serum NfL (sNfL) levels were higher in anti-NF155+ AN than in healthy controls (36.47 vs 7.56 pg/mL, p < 0.001) and correlated with anti-NF155 titers (r = 0.43, p = 0.001), with I-RODS at baseline (r = -0.88, p < 0.001) and with maximum I-RODS achieved (r = -0.58, p = 0.01). Anti-NF155 titers and sNfL levels decreased in all rituximab-treated patients.
Discussion: Anti-NF155 AN presents a distinct clinical profile and good response to rituximab. Autoantibody titers and sNfL are useful to monitor disease status in these patients. The use of untagged-NF155 plasmids minimizes the detection of false anti-NF155+ cases.
Classification of evidence: This study provides Class IV evidence that anti-NF155 antibodies associate with a specific phenotype and response to rituximab.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2019;90(9):981-987. - PubMed
-
- Delmont E, Manso C, Querol L, et al. . Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140(7):1851-1858. - PubMed
-
- Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. . Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73(3):370-380. - PubMed
