The Impact of Age and Genetics on Naltrexone Biotransformation
- PMID: 34728519
- PMCID: PMC9621334
- DOI: 10.1124/dmd.121.000646
The Impact of Age and Genetics on Naltrexone Biotransformation
Abstract
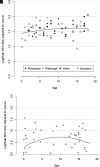
Naltrexone, an opioid antagonist primarily metabolized by aldo-keto reductase 1C4 (AKR1C4), treats pediatric conditions involving compulsiveness (e.g., autism spectrum, Prader-Willi, eating disorders, non-suicidal self-injury). Pharmacokinetic variability is apparent in adults, yet no data are available for children. This study aimed to examine the impact of age and genetic variation on naltrexone biotransformation. Human liver cytosol (HLC) samples (n = 158) isolated from children and adult organ donors were incubated with therapeutically relevant concentrations of naltrexone (0.1, 1 µM). Naltrexone biotransformation was determined by ultraperformance mass spectrometry quantification of the primary metabolite, 6-beta-naltrexol (6βN), and 6βN formation rates (pmol/mg protein/min) were calculated. HLCs from organ donors, age range 0-79 y (mean 16.0 ± 18.2 y), 37% (n = 60) female, 20% (n = 33) heterozygous and 1.2% (n = 2) homozygous for co-occurring AKR1C4 variants (S145C/L311V) showed >200-fold range in 6βN formation (0.37-76.5 pmol/mg protein/min). Source of donor samples was found to be a substantial contributor to variability. Model estimates for a trimmed data set of source-adjusted pediatric samples (aged 0-18 y) suggested that AKR1C4 genetic variation, age, and sex explained 36% of the variability in 6βN formation. Although activity increased steadily from birth and peaked in middle childhood (2-5 years), genetic variation (S145C/L311V) demonstrated a greater effect on activity than did age. Naltrexone biotransformation is highly variable in pediatric and adult livers and can be partly accounted for by individual factors feasible to obtain (e.g., genetic variability, age, sex). These data may inform a precision therapeutics approach (e.g., exposure optimization) to further study Naltrexone responsiveness in children and adults. SIGNIFICANCE STATEMENT: Biotransformation of the commonly used opioid antagonist naltrexone is highly variable and may contribute to reduced therapeutic response. Age, sex, and genetic variation in the drug-metabolizing enzyme, AKR1C4, are potential factors contributing to this variability. In pediatric samples, genetic variation (S145C/L311V) demonstrates a greater impact on activity than age. Additionally, the source of donor samples was identified as an important contributor and must be accounted for to confidently elucidate the biological variables most impactful to drug biotransformation.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Effects of genotype and food on naltrexone exposure in adolescents.Clin Transl Sci. 2022 Nov;15(11):2732-2743. doi: 10.1111/cts.13399. Epub 2022 Oct 5. Clin Transl Sci. 2022. PMID: 36200172 Free PMC article.
-
Effect of food on the pharmacokinetics of oxycodone and naltrexone from ALO-02, an extended release formulation of oxycodone with sequestered naltrexone.Clin Pharmacol Drug Dev. 2015 Sep;4(5):370-6. doi: 10.1002/cpdd.177. Epub 2015 Jan 22. Clin Pharmacol Drug Dev. 2015. PMID: 27137146 Clinical Trial.
-
Naltrexone biotransformation and incidence of subjective side effects: a preliminary study.Alcohol Clin Exp Res. 1997 Aug;21(5):906-9. Alcohol Clin Exp Res. 1997. PMID: 9267542
-
Carbonyl reduction of naltrexone and dolasetron by oxidoreductases isolated from human liver cytosol.J Pharm Pharmacol. 2004 Dec;56(12):1601-6. doi: 10.1211/0022357045020. J Pharm Pharmacol. 2004. PMID: 15563768
-
Opiate antagonists in children and adolescents.Eur Child Adolesc Psychiatry. 2000;9 Suppl 1:I44-50. doi: 10.1007/s007870070018. Eur Child Adolesc Psychiatry. 2000. PMID: 11140779 Review.
Cited by
-
Ontogeny of Scaling Factors for Pediatric Physiologically Based Pharmacokinetic Modeling and Simulation: Cytosolic Protein Per Gram of Liver.Drug Metab Dispos. 2023 Dec;51(12):1578-1582. doi: 10.1124/dmd.123.001417. Epub 2023 Sep 21. Drug Metab Dispos. 2023. PMID: 37735064 Free PMC article.
-
Use of Medications for Opioid Use Disorder in Older Adults.Am J Prev Med. 2025 May;68(5):1015-1021. doi: 10.1016/j.amepre.2025.01.019. Epub 2025 Jan 30. Am J Prev Med. 2025. PMID: 39892523
-
Effective Doses of Low-Dose Naltrexone for Chronic Pain - An Observational Study.J Pain Res. 2024 Mar 21;17:1273-1284. doi: 10.2147/JPR.S451183. eCollection 2024. J Pain Res. 2024. PMID: 38532991 Free PMC article.
-
Delineating gene-environment effects using virtual twins of patients treated with clozapine.CPT Pharmacometrics Syst Pharmacol. 2023 Feb;12(2):168-179. doi: 10.1002/psp4.12886. Epub 2022 Nov 24. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36424701 Free PMC article.
-
Low-Dose Naltrexone as an Adjuvant in Combined Anticancer Therapy.Cancers (Basel). 2024 Mar 21;16(6):1240. doi: 10.3390/cancers16061240. Cancers (Basel). 2024. PMID: 38539570 Free PMC article. Review.
References
-
- Database of Single Nucleotide Polymorphisms (dbSNP). Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine. dbSNP accession:{rs3829125, rs17134592}, (dbSNP Build ID: {GRCh38.p12}). Available from: http://www.ncbi.nlm.nih.gov/SNP/.
-
- Breyer-Pfaff U, Nill K (2004) Carbonyl reduction of naltrexone and dolasetron by oxidoreductases isolated from human liver cytosol. J Pharm Pharmacol 56:1601–1606. - PubMed
-
- Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC (2006) Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res 30:480–490. - PubMed
-
- Feldman HM, Kolmen BK, Gonzaga AM (1999) Naltrexone and communication skills in young children with autism. J Am Acad Child Adolesc Psychiatry 38:587–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

