Triple Targeting of Breast Tumors Driven by Hormonal Receptors and HER2
- PMID: 34728571
- PMCID: PMC8742793
- DOI: 10.1158/1535-7163.MCT-21-0098
Triple Targeting of Breast Tumors Driven by Hormonal Receptors and HER2
Abstract
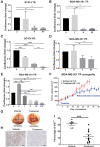
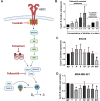
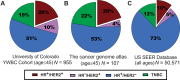
Breast cancers that express hormonal receptors (HR) and HER2 display resistance to targeted therapy. Tumor-promotional signaling from the HER2 and estrogen receptor (ER) pathways converges at the cyclin D1 and cyclin-dependent kinases (CDK) 4 and 6 complex, which drives cell-cycle progression and development of therapeutic resistance. Therefore, we hypothesized that co-targeting of ER, HER2, and CDK4/6 may result in improved tumoricidal activity and suppress drug-resistant subclones that arise on therapy. We tested the activity of the triple targeted combination therapy with tucatinib (HER2 small-molecule inhibitor), palbociclib (CKD4/6 inhibitor), and fulvestrant (selective ER degrader) in HR+/HER2+ human breast tumor cell lines and xenograft models. In addition, we evaluated whether triple targeted combination prevents growth of tucatinib or palbociclib-resistant subclones in vitro and in vivo Triple targeted combination significantly reduced HR+/HER2+ tumor cell viability, clonogenic survival, and in vivo growth. Moreover, survival of HR+/HER2+ cells that were resistant to the third drug in the regimen was reduced by the other two drugs in combination. We propose that a targeted triple combination approach will be clinically effective in the treatment of otherwise drug-resistant tumors, inducing robust responses in patients.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. . Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–30. - PubMed
-
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972–7. - PubMed
-
- Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. . Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 2007;99:694–705. - PubMed
-
- Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. . Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

