Improving properties of the nucleobase analogs T-705/T-1105 as potential antiviral
- PMID: 34728864
- PMCID: PMC8553380
- DOI: 10.1016/bs.armc.2021.08.002
Improving properties of the nucleobase analogs T-705/T-1105 as potential antiviral
Abstract
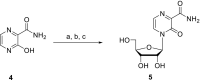
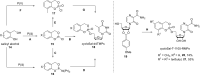
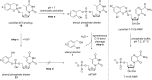
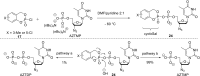
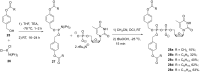
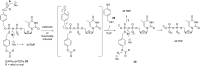
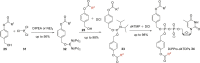
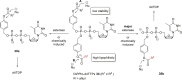
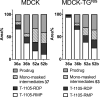
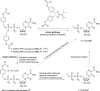
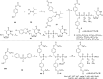
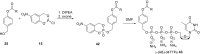
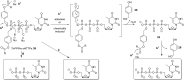
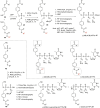
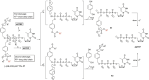
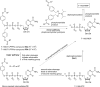
In this minireview we describe our work on the improvement of the nucleobase analogs Favipiravir (T-705) und its non-fluorinated derivative T-1105 as influenza and SARS-CoV-2 active compounds. Both nucleobases were converted into nucleotides and then included in our nucleotide prodrugs technologies cycloSal-monophosphates, DiPPro-nucleoside diphosphates and TriPPPro-nucleoside triphosphates. Particularly the DiPPro-derivatives of T-1105-RDP proved to be very active against influenza viruses. T-1105-derivatives in general were found to be more antivirally active as compared to their T-705 counterpart. This may be due to the low chemical stability of all ribosylated derivatives of T-705. The ribosyltriphosphate derivative of T-1105 was studied for the potential to act as a inhibitor of the SARS-CoV-2 RdRp and was found to be an extremely potent compound causing lethal mutagenesis. The pronucleotide technologies, the chemical synthesis, the biophysical properties and the biological effects of the compounds will be addressed as well.
Keywords: Favipiravir; Influenza; Nucleoside analog; Nucleoside triphosphates; Prodrug; Pronucleotides; RNA polymerases; SARS-CoV-2; TriPPPro.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


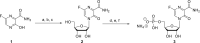



References
-
- Annual Review of Diseases Prioritized Under the Research and Development Blueprint. WHO Research and Development Blueprint; 2018.
-
- Xia H.J., Xie X.P., Shan C., Shi P.Y. Potential Mechanisms for Enhanced Zika Epidemic and Disease. Acs Infect. Dis. 2018;4:656–659. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
