Broad spectrum antiviral nucleosides-Our best hope for the future
- PMID: 34728865
- PMCID: PMC8553659
- DOI: 10.1016/bs.armc.2021.09.001
Broad spectrum antiviral nucleosides-Our best hope for the future
Abstract
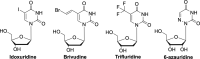
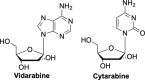
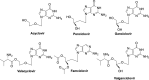
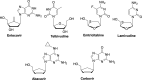
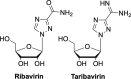
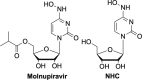
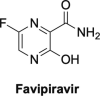
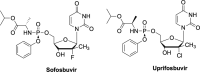
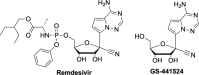
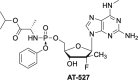
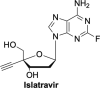
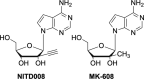
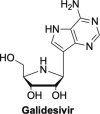
The current focus for many researchers has turned to the development of therapeutics that have the potential for serving as broad-spectrum inhibitors that can target numerous viruses, both within a particular family, as well as to span across multiple viral families. This will allow us to build an arsenal of therapeutics that could be used for the next outbreak. In that regard, nucleosides have served as the cornerstone for antiviral therapy for many decades. As detailed herein, many nucleosides have been shown to inhibit multiple viruses due to the conserved nature of many viral enzyme binding sites. Thus, it is somewhat surprising that up until very recently, many researchers focused more on "one bug one drug," rather than trying to target multiple viruses given those similarities. This attitude is now changing due to the realization that we need to be proactive rather than reactive when it comes to combating emerging and reemerging infectious diseases. A brief summary of prominent nucleoside analogues that previously exhibited broad-spectrum activity and are now under renewed interest, as well as new analogues, that are currently under investigation against SARS-CoV-2 and other viruses is discussed herein.
Keywords: Antiviral; Broad-spectrum; Nucleosides; Pandemic; SARS-CoV-2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
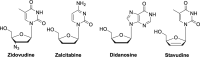
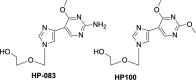
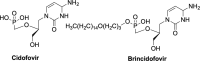
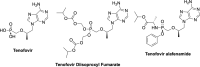


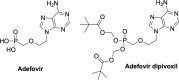
References
-
- Park S.-J., Yu K.-M., Kim Y.-I., Kim S.-M., Kim E.-H., Kim S.-G., Kim E.J., Casel M.A.B., Rollon R., Jang S.-G., Lee M.-H., Chang J.-H., Song M.-S., Jeong H.W., Choi Y., Chen W., Shin W.-J., Jung J.U., Choi Y.K. Antiviral Efficacies of FDA-Approved Drugs Against SARS-CoV-2 Infection in Ferrets. mBio. 2020;11 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
