AL Amyloidosis: Current Chemotherapy and Immune Therapy Treatment Strategies: JACC: CardioOncology State-of-the-Art Review
- PMID: 34729520
- PMCID: PMC8543128
- DOI: 10.1016/j.jaccao.2021.09.003
AL Amyloidosis: Current Chemotherapy and Immune Therapy Treatment Strategies: JACC: CardioOncology State-of-the-Art Review
Abstract
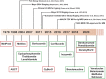
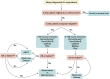
Immunoglobulin light chain (AL) amyloidosis is an incurable plasma cell disorder characterized by deposition of fibrils of misfolded immunoglobulin free light chains (FLC) in target organs, leading to failure. Cardiac involvement is common in AL amyloidosis and represents the single most adverse prognostic feature. A high index of clinical suspicion with rapid tissue diagnosis and commencement of combinatorial, highly effective cytoreductive therapy is crucial to arrest the process of amyloid deposition and preserve organ function. The clinical use of molecularly targeted drugs, such as proteasome inhibitors and immunomodulatory agents, monoclonal antibodies such as daratumumab, and risk-adjusted autologous stem cell transplant in eligible patients, has radically changed the natural history of AL amyloidosis. Here, we review the state-of-the-art treatment landscape in AL amyloidosis with an eye toward future therapeutic venues to impact the outcome of this devastating illness.
Keywords: AL amyloidosis; AL, immunoglobulin light chain; ASCT, autologous stem cell transplant; FLC, free light chains; Ig, immunoglobulin; MM, multiple myeloma; PC, plasma cell; cardiomyopathy; chemotherapy; fibrils; immunotherapy; organ failure; plasma cell disorders.
© 2021 The Authors.
Conflict of interest statement
This work was supported in part by National Institutes of Health/National Institute of Aging grant R21-AG070502-01 (to Dr Comenzo). Dr Bianchi has participated in advisory boards (with personal payment) for Pfizer and Karyopharm. Dr Comenzo has received steering committee fees from Janssen Biotech; has received advisory board fees from Karyopharm Therapeutics; has received fees for serving on a data and safety monitoring committee from Sanofi; and holds patent WO2016187546A1 on anti-CD38 antibodies for treatment of light-chain amyloidosis and other CD38-positive hematologic cancers. Dr Zhang has reported that he has no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Merlini G., Dispenzieri A., Sanchorawala V. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. - PubMed
-
- Bianchi G., Kumar S. Systemic amyloidosis due to clonal plasma cell diseases. Hematol Oncol Clin North Am. 2020;34:1009–1026. - PubMed
-
- Comenzo R.L., Zhang Y., Martinez C., Osman K., Herrera G.A. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. - PubMed
-
- Wyatt A.R., Yerbury J.J., Dabbs R.A., Wilson M.R. Roles of extracellular chaperones in amyloidosis. J Mol Biol. 2012;421:499–516. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

