This is a preprint.
Boosting of Cross-Reactive Antibodies to Endemic Coronaviruses by SARS-CoV-2 Infection but not Vaccination with Stabilized Spike
- PMID: 34729565
- PMCID: PMC8562549
- DOI: 10.1101/2021.10.27.21265574
Boosting of Cross-Reactive Antibodies to Endemic Coronaviruses by SARS-CoV-2 Infection but not Vaccination with Stabilized Spike
Update in
-
Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike.Elife. 2022 Mar 15;11:e75228. doi: 10.7554/eLife.75228. Elife. 2022. PMID: 35289271 Free PMC article.
Abstract
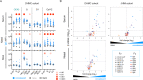
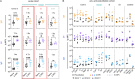
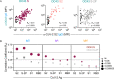
Pre-existing antibodies to endemic coronaviruses (CoV) that cross-react with SARS-CoV-2 have the potential to influence the antibody response to COVID-19 vaccination and infection for better or worse. In this observational study of mucosal and systemic humoral immunity in acutely infected, convalescent, and vaccinated subjects, we tested for cross reactivity against endemic CoV spike (S) protein at subdomain resolution. Elevated responses, particularly to the β-CoV OC43, were observed in all natural infection cohorts tested and were correlated with the response to SARS-CoV-2. The kinetics of this response and isotypes involved suggest that infection boosts preexisting antibody lineages raised against prior endemic CoV exposure that cross react. While further research is needed to discern whether this recalled response is desirable or detrimental, the boosted antibodies principally targeted the better conserved S2 subdomain of the viral spike and were not associated with neutralization activity. In contrast, vaccination with a stabilized spike mRNA vaccine did not robustly boost cross-reactive antibodies, suggesting differing antigenicity and immunogenicity. In sum, this study provides evidence that antibodies targeting endemic CoV are robustly boosted in response to SARS-CoV-2 infection but not to vaccination with stabilized S, and that depending on conformation or other factors, the S2 subdomain of the spike protein triggers a rapidly recalled, IgG-dominated response that lacks neutralization activity.
Figures




References
-
- Coronavirus disease (COVID-19) pandemic, <https://www.who.int/emergencies/diseases/novel-coronavirus-2019> (2021).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
