High Effectiveness of Broad Access Direct-Acting Antiviral Therapy for Hepatitis C in an Australian Real-World Cohort: The REACH-C Study
- PMID: 34729957
- PMCID: PMC8870316
- DOI: 10.1002/hep4.1826
High Effectiveness of Broad Access Direct-Acting Antiviral Therapy for Hepatitis C in an Australian Real-World Cohort: The REACH-C Study
Abstract
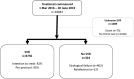
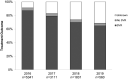
Australia was one of the first countries with unrestricted access to government subsidized direct-acting antiviral (DAA) therapy for adults with chronic hepatitis C virus. This study assessed real-world DAA treatment outcomes across a diverse range of Australian clinical services and evaluated factors associated with successful treatment and loss to follow-up. Real-world Effectiveness of Antiviral therapy in Chronic Hepatitis C (REACH-C) consisted a national observational cohort of 96 clinical services including specialist clinics and less traditional settings such as general practice. Data were obtained on consecutive individuals who commenced DAAs from March 2016 to June 2019. Effectiveness was assessed by sustained virological response ≥12 weeks following treatment (SVR) using intention-to-treat (ITT) and per-protocol (PP) analyses. Within REACH-C, 10,843 individuals initiated DAAs (male 69%; ≥50 years 52%; cirrhosis 22%). SVR data were available in 85% (9,174 of 10,843). SVR was 81% (8,750 of 10,843) by ITT and 95% (8,750 of 9,174) by PP. High SVR (≥92%) was observed across all service types and participant characteristics. Male gender (adjusted odds ratio [aOR] 0.56, 95% confidence interval [CI] 0.43-0.72), cirrhosis (aOR 0.52, 95% CI 0.41-0.64), recent injecting drug use (IDU; aOR 0.64, 95% CI 0.46-0.91) and previous DAA treatment (aOR 0.50, 95% CI 0.28-0.90) decreased the likelihood of achieving SVR. Multiple factors modified the likelihood of loss to follow-up including IDU ± opioid agonist therapy (OAT; IDU only: aOR 1.75, 95% CI 1.44-2.11; IDU + OAT: aOR 1.39, 95% CI 1.11-1.74; OAT only, aOR 1.36; 95% CI 1.13-1.68) and age (aOR 0.97, 95% CI 0.97-0.98). Conclusion: Treatment response was high in a diverse population and through a broad range of services following universal access to DAA therapy. Loss to follow-up presents a real-world challenge. Younger people who inject drugs were more likely to disengage from care, requiring innovative strategies to retain them in follow-up.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures
References
-
- The Kirby Institute . Annual Surveillance Report of HIV, viral hepatitis, STIs 2016. Kensington, NSW, Australia: The Kirby Institute; 2016, 63, 65, 122. https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Annual‐S.... Accessed 15 April 2020.
-
- Ward RP, Kugelmas M. Using pegylated interferon and ribavirin to treat patients with chronic hepatitis C. Am Fam Physician 2005;72:655‐662. - PubMed
-
- Dore GJ, Hajarizadeh B. Elimination of hepatitis C virus in Australia: laying the foundation. Infect Dis Clin North Am 2018;32:269‐279. - PubMed
-
- Australian Government Department of Health . National Hepatitis C Testing Policy. Sydney, Australia; 2020. http://testingportal.ashm.org.au/files/National_Hepatitis_C_Testing_Poli.... Accessed 12 April 2021.
-
- Razali K, Thein HH, Bell J, Cooper‐Stanbury M, Dolan K, Dore G, et al. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend 2007;91:228‐235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous