Effect of Treatment With Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial
- PMID: 34730769
- PMCID: PMC8567189
- DOI: 10.1001/jamacardio.2021.4567
Effect of Treatment With Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial
Abstract
Importance: The use of sacubitril/valsartan is not endorsed by practice guidelines for use in patients with New York Heart Association class IV heart failure with a reduced ejection fraction because of limited clinical experience in this population.
Objective: To compare treatment with sacubitril/valsartan treatment with valsartan in patients with advanced heart failure and a reduced ejection fraction and recent New York Heart Association class IV symptoms.
Design, setting, and participants: A double-blind randomized clinical trial was conducted; a total of 335 patients with advanced heart failure were included. The trial began on March 2, 2017, and was stopped early on March 23, 2020, owing to COVID-19 risk.
Intervention: Patients were randomized to receive sacubitril/valsartan (target dose, 200 mg twice daily) or valsartan (target dose, 160 mg twice daily) in addition to recommended therapy.
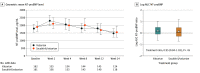
Main outcomes and measures: The area under the curve (AUC) for the ratio of N-terminal pro-brain natriuretic peptide (NT-proBNP) compared with baseline measured through 24 weeks of therapy.
Results: Of the 335 patients included in the analysis, 245 were men (73%); mean (SD) age was 59.4 (13.5) years. Seventy-two eligible patients (18%) were not able to tolerate sacubitril/valsartan, 100 mg/d, during the short run-in period, and 49 patients (29%) discontinued sacubitril/valsartan during the 24 weeks of the trial. The median NT-proBNP AUC for the valsartan treatment arm (n = 168) was 1.19 (IQR, 0.91-1.64), whereas the AUC for the sacubitril/valsartan treatment arm (n = 167) was 1.08 (IQR, 0.75-1.60). The estimated ratio of change in the NT-proBNP AUC was 0.95 (95% CI 0.84-1.08; P = .45). Compared with valsartan, treatment with sacubitril/valsartan did not improve the clinical composite of number of days alive, out of hospital, and free from heart failure events. Aside from a statistically significant increase in non-life-threatening hyperkalemia in the sacubitril/valsartan arm (28 [17%] vs 15 [9%]; P = .04), there were no observed safety concerns.
Conclusions and relevance: The findings of this trial showed that, in patients with chronic advanced heart failure with a reduced ejection fraction, there was no statistically significant difference between sacubitril/valsartan and valsartan with respect to reducing NT-proBNP levels.
Trial registration: ClinicalTrials.gov Identifier: NCT02816736.
Conflict of interest statement
Figures

Comment in
-
Could Neprilysin Be Already Inhibited by BNP in the LIFE Trial?-Reply.JAMA Cardiol. 2022 Jun 1;7(6):657-658. doi: 10.1001/jamacardio.2022.0787. JAMA Cardiol. 2022. PMID: 35507336 No abstract available.
-
Could Neprilysin Be Already Inhibited by BNP in the LIFE Trial?JAMA Cardiol. 2022 Jun 1;7(6):656-657. doi: 10.1001/jamacardio.2022.0784. JAMA Cardiol. 2022. PMID: 35507360 No abstract available.
References
-
- Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68(13):1476-1488. doi: 10.1016/j.jacc.2016.05.011 - DOI - PubMed
-
- Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

