Borophenes made easy
- PMID: 34731005
- PMCID: PMC8565903
- DOI: 10.1126/sciadv.abk1490
Borophenes made easy
Abstract
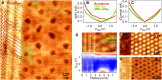
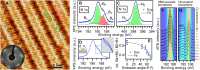
To date, the scalable synthesis of elemental two-dimensional materials beyond graphene still remains elusive. Here, we introduce a versatile chemical vapor deposition (CVD) method to grow borophenes, as well as borophene heterostructures, by selectively using diborane originating from traceable byproducts of borazine. Specifically, metallic borophene polymorphs were successfully synthesized on Ir(111) and Cu(111) single-crystal substrates and conjointly with insulating hexagonal boron nitride (hBN) to form atomically precise lateral borophene-hBN interfaces or vertical van der Waals heterostructures. Thereby, borophene is protected from immediate oxidation by a single hBN overlayer. The ability to synthesize high-quality borophenes with large single-crystalline domains in the micrometer scale by a straight-forward CVD approach opens up opportunities for the study of their fundamental properties and for device incorporation.
Figures


References
-
- Mannix A. J., Kiraly B., Hersam M. C., Guisinger N. P., Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 1, 0014 (2017).
-
- Molle A., Goldberger J., Houssa M., Xu Y., Zhang S.-C., Akinwande D., Buckled two-dimensional Xene sheets. Nat. Mater. 16, 163–169 (2017). - PubMed
-
- Mannix A. J., Zhang Z., Guisinger N. P., Yakobson B. I., Hersam M. C., Borophene as a prototype for synthetic 2D materials development. Nat. Nanotechnol. 13, 444–450 (2018). - PubMed
-
- Feng B., Zhang J., Ito S., Arita M., Cheng C., Chen L., Wu K., Komori F., Sugino O., Miyamoto K., Okuda T., Meng S., Matsuda I., Discovery of 2D anisotropic Dirac cones. Adv. Mater. 30, 1704025 (2018). - PubMed
-
- Zhang Z., Yang Y., Penev E. S., Yakobson B. I., Elasticity, flexibility, and ideal strength of borophenes. Adv. Func. Mat. 27, 1605059 (2017).
LinkOut - more resources
Full Text Sources

