Myoclonic status epilepticus and cerebellar hypoplasia associated with a novel variant in the GRIA3 gene
- PMID: 34731330
- PMCID: PMC8782781
- DOI: 10.1007/s10048-021-00666-1
Myoclonic status epilepticus and cerebellar hypoplasia associated with a novel variant in the GRIA3 gene
Erratum in
-
Correction to: Myoclonic status epilepticus and cerebellar hypoplasia associated with a novel variant in the GRIA3 gene.Neurogenetics. 2022 Jan;23(1):81. doi: 10.1007/s10048-021-00678-x. Neurogenetics. 2022. PMID: 34837146 Free PMC article. No abstract available.
Abstract
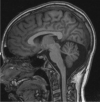
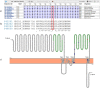
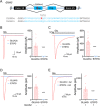
AMPA-type glutamate receptors (AMPARs) are postsynaptic ionotropic receptors which mediate fast excitatory currents. AMPARs have a heterotetrameric structure, variably composed by the four subunits GluA1-4 which are encoded by genes GRIA1-4. Increasing evidence support the role of pathogenic variants in GRIA1-4 genes as causative for syndromic intellectual disability (ID). We report an Italian pedigree where some male individuals share ID, seizures and facial dysmorphisms. The index subject was referred for severe ID, myoclonic seizures, cerebellar signs and short stature. Whole exome sequencing identified a novel variant in GRIA3, c.2360A > G, p.(Glu787Gly). The GRIA3 gene maps to chromosome Xq25 and the c.2360A > G variant was transmitted by his healthy mother. Subsequent analysis in the family showed a segregation pattern compatible with the causative role of this variant, further supported by preliminary functional insights. We provide a detailed description of the clinical evolution of the index subjects and stress the relevance of myoclonic seizures and cerebellar syndrome as cardinal features of his presentation.
Keywords: AMPARs; Cerebellar hypoplasia; GRIA3; Glutamate; Myoclonic status epilepticus.
© 2021. The Author(s).
Figures