Can In Vitro-In Vivo Extrapolation Be Successful? Recognizing the Incorrect Clearance Assumptions
- PMID: 34731496
- PMCID: PMC9542026
- DOI: 10.1002/cpt.2482
Can In Vitro-In Vivo Extrapolation Be Successful? Recognizing the Incorrect Clearance Assumptions
Erratum in
-
Erratum.Clin Pharmacol Ther. 2022 Aug;112(2):411. doi: 10.1002/cpt.2693. Epub 2022 Jul 11. Clin Pharmacol Ther. 2022. PMID: 35816675 No abstract available.
Abstract
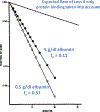
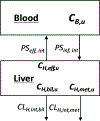
For a number of years, our laboratory has been investigating the underlying reasons for the published poor in vitro-in vivo extrapolation (IVIVE) predictability of human clearance both from a theoretical and from an experimental perspective. Here, we critically examine clearance concepts and commonly employed IVIVE approaches, concluding that there is no theoretical reason that IVIVE should work, just as it does not. Our analysis, however, has identified 10 misconceptions and/or poorly understood aspects of clearance that are listed in the Conclusion section of this manuscript. Chief among these are that all published human drug clearance values are arterial clearances-clearance calculated as organ blood flow multiplied by the extraction ratio is the arterial clearance of the organ of elimination (and not the published drug clearance value)-and that the well-stirred model equation taught in all pharmacokinetic courses that relates organ blood flow, fraction unbound in blood, and intrinsic clearance has no validity. We further list 10 conclusions relating to the IVIVE process. The primary IVIVE-related conclusions are that the intrinsic clearance value determined from an in vitro incubation is an arterial intrinsic clearance, there is no theoretical basis upon which an arterial intrinsic clearance can be related to a whole-body arterial clearance to accomplish IVIVE, there are no published data demonstrating that in vitro intrinsic metabolic clearance can predict in vivo organ clearance as IVIVE assumes, and the scientific basis for the hypothesized albumin-mediated hepatic uptake phenomenon is invalid. We further propose three IVIVE process recommendations.
© 2021 The Authors. Clinical Pharmacology & Therapeutics © 2021 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Figures







Comment in
-
Hepatic clearance models and IVIVE predictions.Clin Pharmacol Ther. 2022 Jun;111(6):1205-1207. doi: 10.1002/cpt.2525. Epub 2022 Mar 1. Clin Pharmacol Ther. 2022. PMID: 35234281 No abstract available.
References
-
- Wood FL, Houston JB, & Hallifax D Clearance prediction methodology needs fundamental improvement: trends common to rat and human hepatocytes/microsomes and implications for experimental methodology. Drug Metab. Dispos. 45, 1178–1188 (2017). - PubMed
-
- Obach RS Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 27, 1350–1359 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

