Mechanochemically-Assisted Synthesis of Polyimides
- PMID: 34731534
- PMCID: PMC9299604
- DOI: 10.1002/cssc.202101975
Mechanochemically-Assisted Synthesis of Polyimides
Abstract
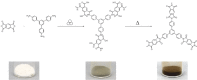
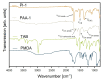
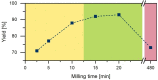
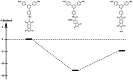
Polyimides were obtained in 99 % yield in under 1 h through the "beat and heat" approach, involving solvent-free vibrational ball milling and a thermal treatment step. The influence of a plethora of additives was explored, such as Lewis acids, Lewis bases, and dehydrating agents, and the mechanochemical reaction was identified to run via a polyamic acid intermediate. The protocol was adopted to a range of substrates inaccessible through solution-based processes, including perylene tetracarboxylic acid dianhydride and melamine. Furthermore, quantum chemical calculations were conducted to identify the water removal as the crucial step in the reaction mechanism. The presented method is substantially faster and more versatile than the solution-based process.
Keywords: beat and heat; green chemistry; mechanochemistry; polymers; solvent-free.
© 2021 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Meador M. A., Annu. Rev. Mater. Sci. 1998, 28, 599.
-
- Chopin S., Chaignon F., Blart E., Odobel F., J. Mater. Chem. 2007, 17, 4139.
-
- None
-
- Zhang M., Niu H., Wu D., Macromol. Rapid Commun. 2018, 39, 1800141; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

