Expeditious recruitment of circulating memory CD8 T cells to the liver facilitates control of malaria
- PMID: 34731605
- PMCID: PMC8628427
- DOI: 10.1016/j.celrep.2021.109956
Expeditious recruitment of circulating memory CD8 T cells to the liver facilitates control of malaria
Abstract
Circulating memory CD8 T cell trafficking and protective capacity during liver-stage malaria infection remains undefined. We find that effector memory CD8 T cells (Tem) infiltrate the liver within 6 hours after malarial or bacterial infections and mediate pathogen clearance. Tem recruitment coincides with rapid transcriptional upregulation of inflammatory genes in Plasmodium-infected livers. Recruitment requires CD8 T cell-intrinsic LFA-1 expression and the presence of liver phagocytes. Rapid Tem liver infiltration is distinct from recruitment to other non-lymphoid tissues in that it occurs both in the absence of liver tissue resident memory "sensing-and-alarm" function and ∼42 hours earlier than in lung infection by influenza virus. These data demonstrate relevance for Tem in protection against malaria and provide generalizable mechanistic insights germane to control of liver infections.
Keywords: CD8 T cells; liver-stage immunity; malaria.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interests The authors declare no competing interests.
Figures

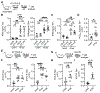



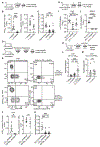
Comment in
-
The Batman and Robin of liver-stage immunity to malaria.Trends Parasitol. 2022 Jan;38(1):7-8. doi: 10.1016/j.pt.2021.11.002. Epub 2021 Nov 24. Trends Parasitol. 2022. PMID: 34836817
References
-
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, and Schumacher TN (2014). T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105. - PubMed
-
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, and Harty JT (2005). Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med 11, 748–756. - PubMed
-
- Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, Fonseca R, Burbach BJ, Hickman HD, Vezys V, et al. (2018). Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol 19, 173–182. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM134880/GM/NIGMS NIH HHS/United States
- T32 AI007485/AI/NIAID NIH HHS/United States
- R01 AI100527/AI/NIAID NIH HHS/United States
- T32 GM139776/GM/NIGMS NIH HHS/United States
- R01 AI127481/AI/NIAID NIH HHS/United States
- R01 AI085515/AI/NIAID NIH HHS/United States
- R01 AI125446/AI/NIAID NIH HHS/United States
- R01 AI114543/AI/NIAID NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- R01 AI042767/AI/NIAID NIH HHS/United States
- T32 AI007511/AI/NIAID NIH HHS/United States
- R21 AI042767/AI/NIAID NIH HHS/United States
- R37 AI042767/AI/NIAID NIH HHS/United States
- T32 AI007343/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

