Epithelial-myeloid exchange of MHC class II constrains immunity and microbiota composition
- PMID: 34731608
- PMCID: PMC9012449
- DOI: 10.1016/j.celrep.2021.109916
Epithelial-myeloid exchange of MHC class II constrains immunity and microbiota composition
Abstract
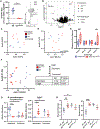
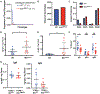
Intestinal epithelial cells (IECs) have long been understood to express high levels of major histocompatibility complex class II (MHC class II) molecules but are not considered canonical antigen-presenting cells, and the impact of IEC-MHC class II signaling on gut homeostasis remains enigmatic. As IECs serve as the primary barrier between underlying host immune cells, we reasoned that IEC-intrinsic antigen presentation may play a role in responses toward the microbiota. Mice with an IEC-intrinsic deletion of MHC class II (IECΔMHC class II) are healthy but have fewer microbial-bound IgA, regulatory T cells (Tregs), and immune repertoire selection. This was associated with increased interindividual microbiota variation and altered proportions of two taxa in the ileum where MHC class II on IECs is highest. Intestinal mononuclear phagocytes (MNPs) have similar MHC class II transcription but less surface MHC class II and are capable of acquiring MHC class II from IECs. Thus, epithelial-myeloid interactions mediate development of adaptive responses to microbial antigens within the gastrointestinal tract.
Keywords: H2-Ab1; MHC class II; antigen processing; dendritic cell; immunity; intestinal epithelia; macrophage; microbiome.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Anderson MJ (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. Published online June 28, 2008. 10.1111/j.1442-9993.2001.01070.pp.x. - DOI
-
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI138945/AI/NIAID NIH HHS/United States
- S10 RR026802/RR/NCRR NIH HHS/United States
- R01 AI123106/AI/NIAID NIH HHS/United States
- S10 OD026959/OD/NIH HHS/United States
- R21 AI142409/AI/NIAID NIH HHS/United States
- R01 DK124336/DK/NIDDK NIH HHS/United States
- K22 AI123481/AI/NIAID NIH HHS/United States
- R01 AT011423/AT/NCCIH NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
- DP2 AT008746/AT/NCCIH NIH HHS/United States
- F30 CA260977/CA/NCI NIH HHS/United States
- R01 DK124317/DK/NIDDK NIH HHS/United States
- R01 AI155887/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

