Study protocol for an open labelled randomised controlled trial of perioperative oral nutrition supplement in breast and colorectal cancer patients undergoing elective surgery
- PMID: 34732233
- PMCID: PMC8565021
- DOI: 10.1186/s13063-021-05716-5
Study protocol for an open labelled randomised controlled trial of perioperative oral nutrition supplement in breast and colorectal cancer patients undergoing elective surgery
Abstract
Background: While it is well established that perioperative use of oral nutrition supplement (ONS) improves nutrition status among severely malnourished surgical cancer patients, the evidence requires further substantiation for non-severely malnourished patients with cancer. This protocol paper presents the rationale and design of a randomised controlled trial to evaluate the effectiveness of preoperative as well as an extended 90-day postoperative use of ONS on nutritional and clinical outcomes among patients undergoing elective surgery for breast and colorectal cancer.
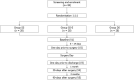
Methods: Patients with primary breast and colorectal cancer undergoing elective surgery are recruited from two tertiary hospitals. Eligible patients are assigned into one of the three intervention arms: (i) Group SS will receive ONS in addition to their normal diet up to 14 days preoperatively and postoperatively up to discharge; (ii) Group SS-E will receive ONS in addition to their normal diet up to 14 days preoperatively, postoperatively up to discharge and for an extended 90 days after discharge; and (iii) Group DS will receive ONS in addition to their normal diet postoperatively up to discharge from the hospital. The ONS is a standard formula fortified with lactium to aid in sleep for recovery. The primary endpoints include changes in weight, body mass index (BMI), serum albumin and prealbumin levels, while secondary endpoints are body composition (muscle and fat mass), muscle strength (handgrip strength), energy and protein intake, sleep quality, haemoglobin, inflammatory markers (transferrin, high sensitivity C-reactive protein, interleukin-6), stress marker (saliva cortisol), length of hospital stay and postoperative complication rate.
Discussion: This trial is expected to provide evidence on whether perioperative supplementation in breast and colorectal cancer patients presenting with high BMI and not severely malnourished but undergoing the stress of surgery would be beneficial in terms of nutritional and clinical outcomes.
Trial registration: ClinicalTrial.gov NCT04400552. Registered on 22 May 2020, retrospectively registered.
Keywords: Breast cancer; Colorectal cancer; Non-severe malnutrition; Perioperative oral nutrition supplement; Surgical outcomes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Veettil SK, Lim KG, Chaiyakunapruk N, Ching SM, Abu Hassan MR. Colorectal cancer in Malaysia: its burden and implications for a multiethnic country. Asian J Surg [Internet]. 2017;40(6):481–489. Available from: https://doi.org/10.1016/j.asjsur.2016.07.005, - PubMed
-
- Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. 2008;5–15. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials