Rationale and design of the 2 by 2 factorial design GnG-trial: a randomized phase-III study to compare two schedules of gemtuzumab ozogamicin as adjunct to intensive induction therapy and to compare double-blinded intensive postremission therapy with or without glasdegib in older patients with newly diagnosed AML
- PMID: 34732236
- PMCID: PMC8564967
- DOI: 10.1186/s13063-021-05703-w
Rationale and design of the 2 by 2 factorial design GnG-trial: a randomized phase-III study to compare two schedules of gemtuzumab ozogamicin as adjunct to intensive induction therapy and to compare double-blinded intensive postremission therapy with or without glasdegib in older patients with newly diagnosed AML
Abstract
Background: Overall survival remains poor in older patients with acute myeloid leukemia (AML) with less than 10% being alive after 5 years. In recent studies, a significant improvement in event-free, relapse-free and overall survival was shown by adding gemtuzumab ozogamicin (GO), a humanized antibody-drug conjugate directed against CD33, to intensive induction therapy once or in a sequential dosing schedule. Glasdegib, the small-molecule inhibitor of smoothened (SMO), also showed improved overall survival in patients not eligible for intensive chemotherapy when combined with low-dose cytarabine compared to low-dose cytarabine alone. These findings warrant further investigations in the phase III GnG trial.
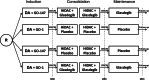
Methods/design: This is a randomized phase III trial with measurable residual disease (MRD) after induction therapy and event-free survival (EFS) as primary endpoints. The two research questions are addressed in a 2 by 2 factorial design. Patients age 60 years and older are upfront randomized 1:1 in one of the two induction arms: GO administered to intensive induction therapy on days 1,4, and 7 versus GO administered once on day 1 (GO-147 versus GO-1), and double-blinded 1:1 in one of the subsequent treatment arms glasdegib vs. placebo as adjunct to consolidation therapy and as single-agent maintenance therapy for six months. Chemotherapy backbone for induction therapy consists of standard 7 + 3 schedule with cytarabine 200 mg/m2 continuously days 1 to 7, daunorubicin 60 mg/m2 days 1, 2, and 3 and high-dose cytarabine (1 g/m2, bi-daily, days 1, 2, and 3) for consolidation therapy. Addressing two primary endpoints, MRD-negativity after induction therapy and event-free survival (EFS), 252 evaluable patients are needed to reject each of the two null hypotheses at a two-sided significance level of 2.5% with a power of at least 85%.
Ethics and dissemination: Ethical approval and approvals from the local and federal competent authorities were granted. Trial results will be reported via peer-reviewed journals and presented at conferences and scientific meetings.
Trial status: Protocol version: 1st version 20.10.2020, no amendments yet. Study initiation on February 16, 2021. First patient was recruited on April 1st.
Trial registration: ClinicalTrials.gov NCT04093505 ; EudraCT 2019-003913-32. Registered on October 30, 2018.
Keywords: acute myeloid leukemia; gemtuzumab ozogamicin; glasdegib; measurable residual disease.
© 2021. The Author(s).
Conflict of interest statement
All authors declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
The funding organizations did not influence the study design nor will they influence the results of this trial.
Figures
References
-
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

