Interchangeable utilization of metals: New perspectives on the impacts of metal ions employed in ancient and extant biomolecules
- PMID: 34732319
- PMCID: PMC8633580
- DOI: 10.1016/j.jbc.2021.101374
Interchangeable utilization of metals: New perspectives on the impacts of metal ions employed in ancient and extant biomolecules
Abstract
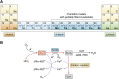
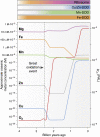
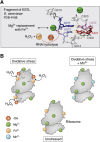
Metal ions provide considerable functionality across biological systems, and their utilization within biomolecules has adapted through changes in the chemical environment to maintain the activity they facilitate. While ancient earth's atmosphere was rich in iron and manganese and low in oxygen, periods of atmospheric oxygenation significantly altered the availability of certain metal ions, resulting in ion replacement within biomolecules. This adaptation mechanism has given rise to the phenomenon of metal cofactor interchangeability, whereby contemporary proteins and nucleic acids interact with multiple metal ions interchangeably, with different coordinated metals influencing biological activity, stability, and toxic potential. The ability of extant organisms to adapt to fluctuating metal availability remains relevant in a number of crucial biomolecules, including the superoxide dismutases of the antioxidant defense systems and ribonucleotide reductases. These well-studied and ancient enzymes illustrate the potential for metal interchangeability and adaptive utilization. More recently, the ribosome has also been demonstrated to exhibit interchangeable interactions with metal ions with impacts on function, stability, and stress adaptation. Using these and other examples, here we review the biological significance of interchangeable metal ions from a new angle that combines both biochemical and evolutionary viewpoints. The geochemical pressures and chemical properties that underlie biological metal utilization are discussed in the context of their impact on modern disease states and treatments.
Keywords: interchangeability; iron; magnesium; manganese; metalloprotein; metals; reactive oxygen species; redox regulation; ribosome; superoxide dismutase.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Bellenger J.P., Wichard T., Xu Y., Kraepiel A.M.L. Essential metals for nitrogen fixation in a free-living N2-fixing bacterium: Chelation, homeostasis and high use efficiency. Environ. Microbiol. 2011;13:1395–1411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

