Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology
- PMID: 34732507
- PMCID: PMC8763186
- DOI: 10.1681/ASN.2021060794
Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology
Abstract
Background: Failure of the glomerular filtration barrier, primarily by loss of slit diaphragm architecture, underlies nephrotic syndrome in minimal change disease. The etiology remains unknown. The efficacy of B cell-targeted therapies in some patients, together with the known proteinuric effect of anti-nephrin antibodies in rodent models, prompted us to hypothesize that nephrin autoantibodies may be present in patients with minimal change disease.
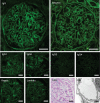
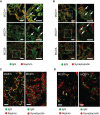
Methods: We evaluated sera from patients with minimal change disease, enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) cohort and from our own institutions, for circulating nephrin autoantibodies by indirect ELISA and by immunoprecipitation of full-length nephrin from human glomerular extract or a recombinant purified extracellular domain of human nephrin. We also evaluated renal biopsies from our institutions for podocyte-associated punctate IgG colocalizing with nephrin by immunofluorescence.
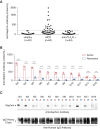
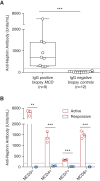
Results: In two independent patient cohorts, we identified circulating nephrin autoantibodies during active disease that were significantly reduced or absent during treatment response in a subset of patients with minimal change disease. We correlated the presence of these autoantibodies with podocyte-associated punctate IgG in renal biopsies from our institutions. We also identified a patient with steroid-dependent childhood minimal change disease that progressed to end stage kidney disease; she developed a massive post-transplant recurrence of proteinuria that was associated with high pretransplant circulating nephrin autoantibodies.
Conclusions: Our discovery of nephrin autoantibodies in a subset of adults and children with minimal change disease aligns with published animal studies and provides further support for an autoimmune etiology. We propose a new molecular classification of nephrin autoantibody minimal change disease to serve as a framework for instigation of precision therapeutics for these patients.
Keywords: autoantibodies; glomerular disease; glomerular filtration barrier; idiopathic nephrotic syndrome; immunology; nephrin; pathology; podocyte; proteinuria.
Copyright © 2022 by the American Society of Nephrology.
Figures


Comment in
-
Authors' Reply.J Am Soc Nephrol. 2022 Mar;33(3):654. doi: 10.1681/ASN.2021121540. Epub 2022 Jan 21. J Am Soc Nephrol. 2022. PMID: 35064049 Free PMC article. No abstract available.
-
Autoimmune Podocytopathies: A Novel Sub-Group of Diseases from Childhood Idiopathic Nephrotic Syndrome.J Am Soc Nephrol. 2022 Mar;33(3):653-654. doi: 10.1681/ASN.2021111469. Epub 2022 Jan 21. J Am Soc Nephrol. 2022. PMID: 35064050 Free PMC article. No abstract available.
References
-
- Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 - PubMed
-
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al. : Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 - PubMed
-
- Liu L, Doné SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, et al. : Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: Insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 10: 2637–2644, 2001 - PubMed
-
- Elie V, Fakhoury M, Deschênes G, Jacqz-Aigrain E: Physiopathology of idiopathic nephrotic syndrome: Lessons from glucocorticoids and epigenetic perspectives. Pediatr Nephrol 27: 1249–1256, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

