Diagnostic Accuracy of Dried Plasma Spot Specimens for HIV-1 Viral Load Testing: A Systematic Review and Meta-analysis
- PMID: 34732684
- PMCID: PMC8826610
- DOI: 10.1097/QAI.0000000000002855
Diagnostic Accuracy of Dried Plasma Spot Specimens for HIV-1 Viral Load Testing: A Systematic Review and Meta-analysis
Abstract
Background: Dried plasma spot specimens may be a viable alternative to traditional liquid plasma in field settings, but the diagnostic accuracy is not well understood.
Methods: Standard databases (PubMed and Medline), conferences, and gray literature were searched until January 2019. The quality of evidence was evaluated using the Standards for Reporting Studies of Diagnostic Accuracy and Quality Assessment of Diagnostic Accuracy Studies-2 criteria. We used univariate and bivariate random effects models to determine misclassification, sensitivity, and specificity across multiple thresholds, overall and for each viral load technology, and to account for between-study variation.
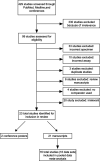
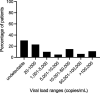
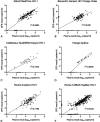
Results: We identified 23 studies for inclusion in the systematic review that compared the diagnostic accuracy of dried plasma spots with that of plasma. Primary data from 16 of the 23 studies were shared and included in the meta-analysis, representing 18 countries, totaling 1847 paired dried plasma spot:plasma data points. The mean bias of dried plasma spot specimens compared with that of plasma was 0.28 log10 copies/mL, whereas the difference in median viral load was 2.25 log10 copies/mL. More dried plasma spot values were undetectable compared with plasma values (43.6% vs. 29.8%). Analyzing all technologies together, the sensitivity and specificity of dried plasma spot specimens were >92% across all treatment failure thresholds compared and total misclassification <5.4% across all treatment failure thresholds compared. Some technologies had lower sensitivity or specificity; however, the results were typically consistent across treatment failure thresholds.
Discussion: Overall, dried plasma spot specimens performed relatively well compared with plasma with sensitivity and specificity values greater than 90% and misclassification rates less than 10% across all treatment failure thresholds reviewed.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Global UNAIDS. HIV & AIDS Statistics—2019 Fact Sheet. Geneva, Switzerland; 2019. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed March 10, 2020.
-
- UNAIDS. 90-90-90. An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014.
-
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. Geneva, Switzerland: World Health Organization; 2016. - PubMed
-
- Clinton Health Access Initiative. HIV market report: the state of HIV treatment, testing, and prevention in low- and middle-income countries. Issue 10, September 2019. Available at: https://3cdmh310dov3470e6x160esb-wpengine.netdna-ssl.com/wp-content/uplo..., Accessed November 30, 2021.

