Health-Related Quality of Life and Toxicity After Definitive High-Dose-Rate Brachytherapy Among Veterans With Prostate Cancer
- PMID: 34733096
- PMCID: PMC8560122
- DOI: 10.12788/fp.0147
Health-Related Quality of Life and Toxicity After Definitive High-Dose-Rate Brachytherapy Among Veterans With Prostate Cancer
Abstract
Purpose: High-dose-rate (HDR) brachytherapy (BT) is a well-tolerated and effective treatment for prostate cancer. There is limited research, however, investigating toxicity outcomes with HDRBT treatment among veterans. The objective of this study is to assess the impact on health-related quality of life (hrQOL) and physician-graded toxicities associated with HDRBT as monotherapy among veterans treated at Edward Hines, Jr. Veterans Affairs Hospital in Hines, Illinois.
Methods: Between 2016 and 2019, 74 veterans with low- or intermediate-risk prostate cancer were treated with HDRBT as monotherapy with 27 Gy in 2 fractions, delivered over 2 implants. Veteran-reported hrQOL in the genitourinary (GU), gastrointestinal (GI), and sexual domains was assessed using the International Prostate Symptoms Score (IPSS) and Expanded Prostate Cancer Index Composite (EPIC-26) questionnaire. Mixed linear effect models were used to assess differences in the hrQOL scores at follow-up compared with baseline scores. Statistically significant differences in hrQOL scores from baseline were further assessed for clinical significance, using minimal clinically important difference (MCID) evaluations.
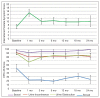
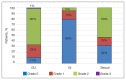
Results: Median follow-up was 18 months. Veterans reported declines in GU, GI, and sexual hrQOL scores immediately after treatment, with the IPSS and EPIC-26 hrQOL scores all displaying significant decrease from baseline over time. The majority of the declines in hrQOL scores met criteria for MCID. These hrQOL scores trended toward a return to baseline, with the EPIC-26 urinary obstruction score returning to baseline at the 18-month follow-up assessment and the EPIC-26 bowel score returning to baseline at the 12-month follow-up. The IPSS, urinary incontinence, and sexual scores did not return to baseline at 18 months. The grade 2 maximum physician-graded GU, GI, and sexual toxicity rates were 65%, 5%, and 53%, respectively. There was 1 incidence of grade 3 GU toxicity but no grade 3 GI or sexual toxicity.
Conclusions: HDRBT as monotherapy is a well-tolerated treatment option for veterans with low- or intermediate-risk prostate cancer, with favorable veteran-reported and physician-graded toxicities. Veterans should be educated about HDRBT as an option when counseled regarding treatment for localized prostate cancer.
Copyright © 2021 Frontline Medical Communications Inc., Parsippany, NJ, USA.
Conflict of interest statement
Author disclosures The authors report no actual or potential conflicts of interest with regard to this article.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous