Is there a preferred first-line therapy for metastatic renal cell carcinoma? A network meta-analysis
- PMID: 34733356
- PMCID: PMC8558789
- DOI: 10.1177/17562872211053189
Is there a preferred first-line therapy for metastatic renal cell carcinoma? A network meta-analysis
Abstract
Background: In recent years, new therapeutic combinations based on immunotherapy provided significant benefits as a first-line treatment for patients with advanced renal cell carcinoma (mRCC).
Objective: This work aims to address the lack of head-to-head comparisons and the uncertainty of the benefit from immunotherapy-based combinations in all the International Metastatic RCC Database Consortium (IMDC) subgroups.
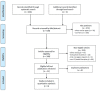
Design setting and participants: A systematic review and a network meta-analysis were performed. Overall survival (OS) in the intention-to-treat (ITT) population was the primary endpoint. OS according to IMDC subgroups (favorable, intermediate, poor), PD-L1 expression, and grade ⩾3 adverse events (AEs) were secondary endpoints. A SUCRA analysis was performed.
Results and limitations: Six randomized phase III trials with 5121 patients were included. There was a high likelihood (82%) that nivolumab-cabozantinib was the preferred treatment in OS. The benefit of ICI-based combinations over sunitinib was unclear in the favorable-risk subgroup. Nivolumab-ipilimumab had the best risk/benefit ratio among all the ICI-based combinations. The limitations were the lack of individual patient data; the heterogeneity of patients' characteristics, trial designs, and follow-up times; and a limited number of studies for indirect comparisons.
Conclusions: A customized approach for the first-line treatment of patients with mRCC should consider the risk/benefit profile of each treatment option, especially considering the likeliness of long-term survival finally reached in this setting.
Keywords: first-line; immune checkpoint inhibitors; meta-analysis; renal cell carcinoma; tyrosine kinase inhibitors.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.C. received Travel/Accommodation/Expenses from Novartis, Pfizer, Janssen, and Ipsen; advisory board from Janssen. C.M. received honoraria as a speaker for scientific events from Astellas and Ipsen. S.B. received honoraria as a speaker at scientific events and advisory role from Bristol-Myers Squibb (BMS), Pfizer, MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca, and Novartis; he also received research funding from Novartis. O.C. received honoraria as advisor/speaker from Astellas, AstraZeneca, Bayer, Janssen, MSD, Pfizer, and Sanofi. A.G. has declared consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI, and Daichii Sankyo; Speakers Bureau from Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, Astra Zeneca, Celgene, and Daichii Sankyo; and research funds from EISAI, Eli Lilly, and Roche. M.B. received research funding from Roche S.p.A., Seqirus UK, Pfizer, Novartis, BMS, Astra Zeneca, and Sanofi Genzyme; honoraria as a speaker at scientific events from Bristol-Myers Squibb (BMS), Novartis, Astra Zeneca, Pierre Fabre, and Pfizer; fees as a consultant for advisory role from Novartis, BMS, IPSEN, and Pfizer; and fees for copyright transfer and consultancies from Sciclone Pharmaceuticals. All the other authors declare no conflicts of interest.
Figures

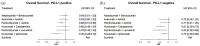
References
-
- Rini BI, Powles T, Atkins MB, et al.. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019; 393: 2404–2415. - PubMed
-
- Rini BI, Plimack ER, Stus V, et al.. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med 2019; 380: 1116–1127. - PubMed
-
- Motzer R, Alekseev B, Rha SY, et al.. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. New Engl J Med 2021; 384: 1289–1300. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

