Maternal malnutrition and anaemia in India: dysregulations leading to the 'thin-fat' phenotype in newborns
- PMID: 34733503
- PMCID: PMC8532069
- DOI: 10.1017/jns.2021.83
Maternal malnutrition and anaemia in India: dysregulations leading to the 'thin-fat' phenotype in newborns
Abstract
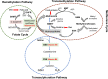
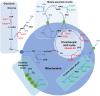
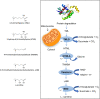
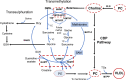
Maternal and child malnutrition and anaemia remain the leading factors for health loss in India. Low birth weight (LBW) offspring of women suffering from chronic malnutrition and anaemia often exhibit insulin resistance and infantile stunting and wasting, together with increased risk of developing cardiometabolic disorders in adulthood. The resulting self-perpetuating and highly multifactorial disease burden cannot be remedied through uniform dietary recommendations alone. To inform approaches likely to alleviate this disease burden, we implemented a systems-analytical approach that had already proven its efficacy in multiple published studies. We utilised previously published qualitative and quantitative analytical results of rural and urban field studies addressing maternal and infantile metabolic and nutritional parameters to precisely define the range of pathological phenotypes encountered and their individual biological characteristics. These characteristics were then integrated, via extensive literature searches, into metabolic and physiological mechanisms to identify the maternal and foetal metabolic dysregulations most likely to underpin the 'thin-fat' phenotype in LBW infants and its associated pathological consequences. Our analyses reveal hitherto poorly understood maternal nutrition-dependent mechanisms most likely to promote and sustain the self-perpetuating high disease burden, especially in the Indian population. This work suggests that it most probably is the metabolic consequence of 'ill-nutrition' - the recent and rapid dietary shifts to high salt, high saturated fats and high sugar but low micronutrient diets - over an adaptation to 'thrifty metabolism' which must be addressed in interventions aiming to significantly alleviate the leading risk factors for health deterioration in India.
Keywords: 5-mTHF, 5-methyltetrahydrofolate; Anaemia; BAT, brown adipocyte tissue; EAA, essential amino acids; FA, fatty acid; GSH, glutathione; Hcy, homocysteine; LBW, low birth weight; Low birth weight; Malnutrition; PE, phosphatidylethanolamine; Pathological mechanisms; Physiological programming; SAM, S-adenosyl methionine; TG, triacylglycerol; WAT, white adipocyte tissue.
© The Author(s) 2021.
Figures
Similar articles
-
The burden of child and maternal malnutrition and trends in its indicators in the states of India: the Global Burden of Disease Study 1990-2017.Lancet Child Adolesc Health. 2019 Dec;3(12):855-870. doi: 10.1016/S2352-4642(19)30273-1. Epub 2019 Sep 18. Lancet Child Adolesc Health. 2019. PMID: 31542357 Free PMC article.
-
Risk factors for preterm and term low birthweight in Ahmedabad, India.Int J Epidemiol. 1992 Apr;21(2):263-72. doi: 10.1093/ije/21.2.263. Int J Epidemiol. 1992. PMID: 1428479
-
Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low- and middle-income countries (LMIC).Cochrane Database Syst Rev. 2019 Jun 17;6(6):CD011695. doi: 10.1002/14651858.CD011695.pub2. Cochrane Database Syst Rev. 2019. PMID: 31204795 Free PMC article.
-
Maternal anemia and underweight as determinants of pregnancy outcomes: cohort study in eastern rural Maharashtra, India.BMJ Open. 2018 Aug 8;8(8):e021623. doi: 10.1136/bmjopen-2018-021623. BMJ Open. 2018. PMID: 30093518 Free PMC article.
-
Lipid intake during pregnancy in developing countries: possible effect of essential fatty acid deficiency on fetal growth.Prostaglandins Leukot Essent Fatty Acids. 1993 Feb;48(2):139-42. doi: 10.1016/0952-3278(93)90101-2. Prostaglandins Leukot Essent Fatty Acids. 1993. PMID: 8446650 Review.
Cited by
-
Youth-onset Type 2 Diabetes: An Overview of Pathophysiology, Prognosis, Prevention and Management.Curr Diab Rep. 2024 Aug;24(8):183-195. doi: 10.1007/s11892-024-01546-2. Epub 2024 Jul 3. Curr Diab Rep. 2024. PMID: 38958831 Free PMC article. Review.
-
Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: the pandora wave 1 study.Int J Obes (Lond). 2023 Nov;47(11):1120-1131. doi: 10.1038/s41366-023-01366-6. Epub 2023 Aug 22. Int J Obes (Lond). 2023. PMID: 37608089 Free PMC article.
-
Effects of preconception nutrition interventions on pregnancy and birth outcomes in South Asia: a systematic review.Lancet Reg Health Southeast Asia. 2025 Apr 24;36:100580. doi: 10.1016/j.lansea.2025.100580. eCollection 2025 May. Lancet Reg Health Southeast Asia. 2025. PMID: 40421124 Free PMC article. Review.
References
-
- Simon Iain Hay RAS, Ashkan A, Sanjay A, et al. (2017) India: Health of the Nation's States — The India State-Level Disease Burden Initiative. New Delhi, India: Indian Council of Medical Research.
-
- Yajnik CS (2002) The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev 3, 217–224. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

