Real world effectiveness of benralizumab on respiratory function and asthma control
- PMID: 34733505
- PMCID: PMC8506201
- DOI: 10.4081/mrm.2021.785
Real world effectiveness of benralizumab on respiratory function and asthma control
Abstract
Background: Biological drugs have been recognized as a breakthrough in the treatment of severe refractory asthma. This retrospective real-life observational study aims to evaluate the effect of add-on benralizumab on lung function, exacerbation rate, oral corticosteroids (OCS) reduction and asthma control questionnaire (ACQ) score after 52-weeks.
Methods: In this observational study, a cohort of 18 patients with severe eosinophilic asthma (SEA) according to the ERS / ATS and GINA 2020 classifications, with reference to the Pulmonology Unit of the Azienda USL - IRCCS, Reggio Emilia, Italy, were enrolled from 1 September 2019 to 31 August 2020. For each patient, the following data were collected: demographic data (age, sex, age of onset of asthma, history of smoking and atopy); comorbidity; clinical data (lung function, exacerbations, emergency room visits and hospitalizations); asthma control questionnaire (ACQ); biomarkers (blood eosinophil count and total serum IgE); asthma control drugs as high-dose inhaled corticosteroids / long-acting beta-adrenoceptor agonists (ICS / LABA), long-acting muscarinic antagonists (LAMA), leukotriene receptor antagonists (LTRA), theophylline, OCS. The benralizumab 30 mg treatment schedule was based on the currently recommended dosing regimen.
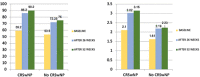
Results: After end-of-treatment (EOT), a complete weaning of all patients from OCS was confirmed. After 26 weeks, the number of exacerbations decreased from 2.90 to 0.05 (p<0.0001), hospitalizations and ACQ score decreased from 3.37 to 0.97 (p<0.0001). At EOT, the number of exacerbations was unchanged, while no hospitalizations had occurred. Overall, lung function markedly improved over the study period. After 52 weeks, the increase in FEV1 from baseline was 26,8% (p=0.0002). The subset of patients with nasal polyposis (NP) had an increase of nearly 50% (1008 ml) and patients with blood eosinophils count (BEC) greater than 500 cells / μl showed an increase of 68% (1081 ml) in FEV1 at EOT.
Conclusions: The notable improvement in respiratory function is a significant result in this study and it is much higher than what has emerged to date. This result, together with the OCS sparing effect and the excellent clinical control of asthma, makes benralizumab a reliable and safe therapeutic option for SEA.
Keywords: Severe refractory asthma; benralizumab; biologics; exacerbations; lung function; oral corticosteroids.
©Copyright: the Author(s).
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous