Critical Updates on Chronic Hepatitis B Virus Infection in 2021
- PMID: 34733599
- PMCID: PMC8557099
- DOI: 10.7759/cureus.19152
Critical Updates on Chronic Hepatitis B Virus Infection in 2021
Abstract
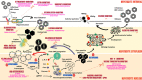
Chronic hepatitis B virus (HBV) infection is a global healthcare burden in the form of chronic liver disease, cirrhosis, liver failure and liver cancer. There is no definite cure for the virus and even though extensive vaccination programs have reduced the burden of liver disease in the future population, treatment options to eradicate the virus from the host are still lacking. In this review, we discuss in detail current updates on the structure and applied biology of the virus in the host, examine updates to current treatment and explore novel and state-of-the-art therapeutics in the pipeline for management of chronic HBV. Furthermore, we also specifically review clinical updates on HBV-related acute on chronic liver failure (ACLF). Current treatments for chronic HBV infection have seen important updates in the form of considerations for treating patients in the immune tolerant phase and some clarity on end points for treatment and decisions on finite therapy with nucleos(t)ide inhibitors. Ongoing cutting-edge research on HBV biology has helped us identify novel target areas in the life cycle of the virus for application of new therapeutics. Due to improvements in the area of genomics, the hope for therapeutic vaccines, vector-based treatments and focused management aimed at targeting host integration of the virus and thereby a total cure could become a reality in the near future. Newer clinical prognostic tools have improved our understanding of timing of specific treatment options for the catastrophic syndrome of ACLF secondary to reactivation of HBV. In this review, we discuss in detail pertinent updates regarding virus biology and novel therapeutic targets with special focus on the appraisal of prognostic scores and treatment options in HBV-related ACLF.
Keywords: aclf; acute hepatitis; acute liver failure; antiviral; chronic hepatitis; cirrhosis; hbv; hepatocellular carcinoma; liver failure; portal hypertension.
Copyright © 2021, Philips et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- When (and when not) to treat patients with HBV infection. Fricker ZP, Reddy KR. Clin Gastroenterol Hepatol. 2019;17:2644–2647. - PubMed
-
- The efficacy of hepatitis B treatments in achieving HBsAg seroclearance: a systematic review and meta-analysis. Fonseca MA, Ling JZ, Al-Siyabi O, Co-Tanko V, Chan E, Lim SG. J Viral Hepat. 2020;27:650–662. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
