I-KID study protocol: evaluation of efficacy, outcomes and safety of a new infant haemodialysis and ultrafiltration machine in clinical use: a randomised clinical investigation using a cluster stepped-wedge design
- PMID: 34734128
- PMCID: PMC8524285
- DOI: 10.1136/bmjpo-2021-001224
I-KID study protocol: evaluation of efficacy, outcomes and safety of a new infant haemodialysis and ultrafiltration machine in clinical use: a randomised clinical investigation using a cluster stepped-wedge design
Abstract
Introduction: The I-KID study aims to determine the clinical efficacy, outcomes and safety of a novel non-CE-marked infant haemodialysis machine, the Newcastle Infant Dialysis Ultrafiltration System (NIDUS), compared with currently available therapy in the UK. NIDUS is specifically designed for renal replacement therapy in small babies between 0.8 and 8 kg.
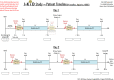
Methods and analysis: The clinical investigation is taking place in six UK centres. This is a randomised clinical investigation using a cluster stepped-wedge design. The study aims to recruit 95 babies requiring renal replacement therapy in paediatric intensive care units over 20 months.
Ethics and dissemination: The study has high parent and public involvement at all stages in its design and parents will be involved in dissemination of results to parents and professionals via publications, conference proceedings and newsletters. The study has has ethics permissions from Tyne and Wear South Research Ethics Committee.
Trial registration numbers: IRAS ID number: 170 481MHRA Reference: CI/2017/0066ISRCT Number: 13 787 486CPMS ID number: 36 558NHS REC reference: 16/NE/0008Eudamed number: CIV-GB-18-02-023105Link to full protocol v6.0: https://fundingawards.nihr.ac.uk/award/14/23/26.
Keywords: neonatology; nephrology; technology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: Dr Malcolm Coulthard is named on the patent and will receive some royalties if the NIDUS device goes into commercial production. The Newcastle upon Tyne NHS Foundation Trust will receive some royalties if the NIDUS device goes into commercial production.Allmed, the device manufacturers, are providing 17 NIDUS devices for loan to sites for the I-KID study and for potential compassionate use in the post-study period. NIDUS consumables are purchased as per normal clinical care. The rest of the authors have completed the COI form and have no competing interests to declare.
Figures
Similar articles
-
Evaluation of efficacy, outcomes and safety of infant haemodialysis and ultrafiltration in clinical use: I-KID a stepped wedge cluster RCT.Southampton (UK): National Institute for Health and Care Research; 2024 Jan. Southampton (UK): National Institute for Health and Care Research; 2024 Jan. PMID: 38232217 Free Books & Documents. Review.
-
Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis.Pediatr Nephrol. 2014 Oct;29(10):1873-81. doi: 10.1007/s00467-014-2923-3. Epub 2014 Aug 15. Pediatr Nephrol. 2014. PMID: 25125229 Free PMC article. Clinical Trial.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Evaluating the impact of a community health worker programme on non-communicable disease, malnutrition, tuberculosis, family planning and antenatal care in Neno, Malawi: protocol for a stepped-wedge, cluster randomised controlled trial.BMJ Open. 2018 Jul 13;8(7):e019473. doi: 10.1136/bmjopen-2017-019473. BMJ Open. 2018. PMID: 30007924 Free PMC article.
-
Appendix to dialysis centre guidelines: recommendations for the relationship between outpatient haemodialysis centres and reference hospitals. Opinions from the Outpatient Dialysis Group. Grupo de Trabajo de Hemodiálisis Extrahospitalaria.Nefrologia. 2011;31(6):664-9. doi: 10.3265/Nefrologia.pre2011.Oct.11001. Nefrologia. 2011. PMID: 22130281 English, Spanish.
Cited by
-
Clinical characteristics and outcomes of continuous renal replacement therapy performed on younger children weighing up to 10 kg.Turk J Med Sci. 2023 Jun;53(3):791-802. doi: 10.55730/1300-0144.5642. Epub 2023 Jun 19. Turk J Med Sci. 2023. PMID: 37476891 Free PMC article.
-
Validation of a low-cost continuous renal replacement therapy dialysate fluid controller for experimental purposes.Intensive Care Med Exp. 2024 Feb 2;12(1):9. doi: 10.1186/s40635-024-00593-z. Intensive Care Med Exp. 2024. PMID: 38302808 Free PMC article.
-
In vitro measurements of ultrafiltration precision in hemofiltration and hemodialysis devices used in infants.Pediatr Nephrol. 2022 Dec;37(12):3189-3194. doi: 10.1007/s00467-022-05439-y. Epub 2022 Mar 29. Pediatr Nephrol. 2022. PMID: 35352191 Free PMC article.
-
Kidney support for babies: building a comprehensive and integrated neonatal kidney support therapy program.Pediatr Nephrol. 2023 Jul;38(7):2043-2055. doi: 10.1007/s00467-022-05768-y. Epub 2022 Oct 13. Pediatr Nephrol. 2023. PMID: 36227440 Review.
-
Safety and efficacy of continuous renal replacement therapy for children less than 10 kg using standard adult machines.Eur J Pediatr. 2023 Aug;182(8):3619-3629. doi: 10.1007/s00431-023-05007-y. Epub 2023 May 26. Eur J Pediatr. 2023. PMID: 37233776 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical