The link between brain acidosis, breathing and seizures: a novel mechanism of action for the ketogenic diet in a model of infantile spasms
- PMID: 34734183
- PMCID: PMC8557655
- DOI: 10.1093/braincomms/fcab189
The link between brain acidosis, breathing and seizures: a novel mechanism of action for the ketogenic diet in a model of infantile spasms
Abstract
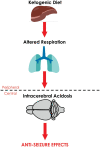
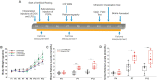
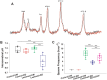
Infantile spasms (IS) syndrome is a catastrophic, epileptic encephalopathy of infancy that is often refractory to current antiepileptic therapies. The ketogenic diet (KD) has emerged as an alternative treatment for patients with medically intractable epilepsy, though the prospective validity and mechanism of action for IS remains largely unexplored. We investigated the KD's efficacy as well as its mechanism of action in a rodent model of intractable IS. The spasms were induced using the triple-hit paradigm and the animals were then artificially reared and put on either the KD (4:1 fats: carbohydrate + protein) or a control milk diet (CM; 1.7:1). 31Phosphorus magnetic resonance spectroscopy (31P MRS) and head-out plethysmography were examined in conjunction with continuous video-EEG behavioural recordings in lesioned animals and sham-operated controls. The KD resulted in a peripheral ketosis observed both in the blood and urine. The KD led to a robust reduction in the frequency of spasms observed, with approximately a 1.5-fold increase in the rate of survival. Intriguingly, the KD resulted in an intracerebral acidosis as measured with 31P MRS. In addition, the respiratory profile of the lesioned rats on the KD was significantly altered with slower, deeper and longer breathing, resulting in decreased levels of expired CO2. Sodium bicarbonate supplementation, acting as a pH buffer, partially reversed the KD's protective effects on spasm frequency. There were no differences in the mitochondrial respiratory profiles in the liver and brain frontal cortex measured between the groups, supporting the notion that the effects of the KD on breathing are not entirely due to changes in intermediary metabolism. Together, our results indicate that the KD produces its anticonvulsant effects through changes in respiration leading to intracerebral acidosis. These findings provide a novel understanding of the mechanisms underlying the anti-seizure effects of the KD in IS. Further research is required to determine whether the effects of the KD on breathing and intracerebral acid-base balance are seen in other paediatric models of epilepsy.
Keywords: acidosis; epilepsy; plethysmography; respiration; spectroscopy.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





Comment on
-
New developments for dietary treatment of epilepsy after a century of history for the ketogenic diet.Brain Commun. 2021 Oct 7;3(4):fcab234. doi: 10.1093/braincomms/fcab234. eCollection 2021. Brain Commun. 2021. PMID: 34704031 Free PMC article.
Similar articles
-
The Efficacy of the Ketogenic Diet in Improving Seizures and EEG Findings in Patients with Refractory Infantile Spasms.Iran J Child Neurol. 2022 Fall;16(4):45-54. doi: 10.22037/ijcn.v16i3.31429. Epub 2022 Oct 23. Iran J Child Neurol. 2022. PMID: 36478996 Free PMC article.
-
Intermittent vs continuous ketogenic diet: Impact on seizures, gut microbiota, and mitochondrial metabolism.Epilepsia. 2023 Aug;64(8):e177-e183. doi: 10.1111/epi.17688. Epub 2023 Jun 29. Epilepsia. 2023. PMID: 37335622
-
Addition of Prebiotics to the Ketogenic Diet Improves Metabolic Profile but Does Not Affect Seizures in a Rodent Model of Infantile Spasms Syndrome.Nutrients. 2022 May 26;14(11):2210. doi: 10.3390/nu14112210. Nutrients. 2022. PMID: 35684010 Free PMC article.
-
Insights into the Cellular Interactions and Molecular Mechanisms of Ketogenic Diet for Comprehensive Management of Epilepsy.Curr Neuropharmacol. 2022;20(11):2034-2049. doi: 10.2174/1570159X20666220420130109. Curr Neuropharmacol. 2022. PMID: 35450526 Free PMC article. Review.
-
How does the ketogenic diet induce anti-seizure effects?Neurosci Lett. 2017 Jan 10;637:4-10. doi: 10.1016/j.neulet.2015.07.034. Epub 2015 Jul 26. Neurosci Lett. 2017. PMID: 26222258 Review.
Cited by
-
Probiotics counteract hepatic steatosis caused by ketogenic diet and upregulate AMPK signaling in a model of infantile epilepsy.EBioMedicine. 2022 Feb;76:103838. doi: 10.1016/j.ebiom.2022.103838. Epub 2022 Feb 9. EBioMedicine. 2022. PMID: 35148983 Free PMC article.
-
The Role of TRPV1 in Febrile Seizure Susceptibility: Inflammation, Respiratory Alkalosis, and Seizure Threshold.Am J Respir Cell Mol Biol. 2024 Aug;71(2):139-140. doi: 10.1165/rcmb.2024-0182ED. Am J Respir Cell Mol Biol. 2024. PMID: 38701493 Free PMC article. No abstract available.
-
Ketogenic Diet Reduces Age-Induced Chronic Neuroinflammation in Mice.Aging Biol. 2024;2:20240038. doi: 10.59368/agingbio.20240038. Epub 2024 Dec 16. Aging Biol. 2024. PMID: 39697898 Free PMC article.
-
A ketogenic diet reduces age-induced chronic neuroinflammation in mice Running title: ketogenic diet and brain inflammaging.bioRxiv [Preprint]. 2023 Dec 4:2023.12.01.569598. doi: 10.1101/2023.12.01.569598. bioRxiv. 2023. Update in: Aging Biol. 2024;2:20240038. doi: 10.59368/agingbio.20240038. PMID: 38106160 Free PMC article. Updated. Preprint.
-
Psychotomimetic compensation versus sensitization.Pharmacol Res Perspect. 2024 Aug;12(4):e1217. doi: 10.1002/prp2.1217. Pharmacol Res Perspect. 2024. PMID: 38923845 Free PMC article. Review.
References
-
- Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L.. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18(3):197–201. - PubMed
-
- Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13(1):14–23. - PubMed
-
- Riikonen R. Infantile spasms: Outcome in clinical studies. Pediatr Neurol. 2020;108:54–64. - PubMed
-
- Krijgh EJC, Catsman-Berrevoets CE, Neuteboom RF.. Early seizure freedom is a prognostic factor for survival in patients with West syndrome. Neuropediatrics. 2018;49(4):279–282. - PubMed
-
- Darke K, Edwards SW, Hancock E, et al.Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: A multi-centre randomised trial. Arch Dis Child. 2010;95(5):382–386. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
