Importance of tumour volume and histology in trimodality treatment of patients with Stage IIIA non-small cell lung cancer-results from a retrospective analysis
- PMID: 34734237
- PMCID: PMC8972331
- DOI: 10.1093/icvts/ivab291
Importance of tumour volume and histology in trimodality treatment of patients with Stage IIIA non-small cell lung cancer-results from a retrospective analysis
Abstract
Objectives: Chemoradiotherapy (CRT) has been the backbone of guideline-recommended treatment for Stage IIIA non-small cell lung cancer (NSCLC). However, in selected operable patients with a resectable tumour, good results have been achieved with trimodality treatment (TT). The objective of this bi-institutional analysis of outcomes in patients treated for Stage IIIA NSCLC was to identify particular factors supporting the role of surgery after CRT.
Methods: In a 2-centre retrospective cohort study, patients with Stage III NSCLC (seventh edition TNM) were identified and those patients with Stage IIIA who were treated with CRT or TT between January 2007 and December 2013 were selected. Patient characteristics as well as tumour parameters were evaluated in relation to outcome and whether or not these variables were predictive for the influence of treatment (TT or CRT) on outcome [overall survival (OS) or progression-free survival (PFS)]. Estimation of treatment effect on PFS and OS was performed using propensity-weighted cox regression analysis based on inverse probability weighting.
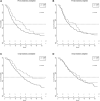
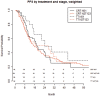
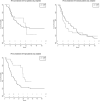
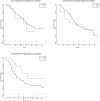
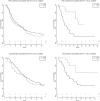
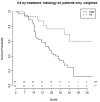
Results: From a database of 725 Stage III NSCLC patients, 257 Stage IIIA NSCLC patients, treated with curative intent, were analysed; 186 (72%) with cIIIA-N2 and 71 (28%) with cT3N1/cT4N0 disease. One hundred and ninety-six (76.3%) patients were treated by CRT alone (high-dose radiation with daily low-dose cisplatin) and 61 (23.7%) by TT. The unweighted data showed that TT resulted in better PFS and OS. After weighting for factors predictive of treatment assignment, patients with a large gross tumour volume (>120 cc) had better PFS when treated with TT, and patients with an adenocarcinoma treated with TT had better OS, regardless of tumour volume.
Conclusions: Patients with Stage IIIA NSCLC and large tumour volume, as well as patients with adenocarcinoma, who were selected for TT, had favourable outcome compared to patients receiving CRT. This information can be used to assist multidisciplinary team decision-making and for stratifying patients in studies comparing TT and definitive CRT.
Keywords: Chemoradiotherapy; Non-small cell lung cancer Stage IIIA; Surgery; Trimodality treatment; Tumour volume.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures
Similar articles
-
Trimodality therapy for stage IIIA non-small cell lung cancer: benchmarking multi-disciplinary team decision-making and function.Lung Cancer. 2014 Aug;85(2):218-23. doi: 10.1016/j.lungcan.2014.06.005. Epub 2014 Jun 16. Lung Cancer. 2014. PMID: 24976333
-
Neoadjuvant treatment followed by surgery versus definitive chemoradiation in stage IIIA-N2 non-small-cell lung cancer: A multi-institutional study by the oncologic group for the study of lung cancer (Spanish Radiation Oncology Society).Lung Cancer. 2018 Apr;118:119-127. doi: 10.1016/j.lungcan.2018.02.008. Epub 2018 Feb 14. Lung Cancer. 2018. PMID: 29571989
-
Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE).J Clin Oncol. 2015 Dec 10;33(35):4194-201. doi: 10.1200/JCO.2015.62.6812. Epub 2015 Nov 2. J Clin Oncol. 2015. PMID: 26527789 Clinical Trial.
-
Stage 3 N2 Lung Cancer: A Multidisciplinary Therapeutic Conundrum.Curr Oncol Rep. 2024 Jan;26(1):65-79. doi: 10.1007/s11912-023-01486-2. Epub 2024 Jan 2. Curr Oncol Rep. 2024. PMID: 38180692 Free PMC article. Review.
-
Multidisciplinary management of N2 stage III non-small cell lung cancer: opportunities and challenges for radiation oncology.Transl Lung Cancer Res. 2025 Mar 31;14(3):991-1006. doi: 10.21037/tlcr-24-974. Epub 2025 Mar 14. Transl Lung Cancer Res. 2025. PMID: 40248721 Free PMC article. Review.
References
-
- Morgensztern MD, Ng SH, Gao F, Govindan R.. Trends in stage distribution for patients with non-small cell lung cancer: a national cancer database survey. J Thorac Oncol 2010;5:29–33. - PubMed
-
- Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J. et al.; ESMO Guidelines Committee. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol 2017;28:iv1–21. - PubMed
-
- Eberhardt WE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V. et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194–201. - PubMed
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al.; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

