Papillary lesions of the breast
- PMID: 34734332
- PMCID: PMC8983543
- DOI: 10.1007/s00428-021-03182-7
Papillary lesions of the breast
Abstract
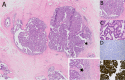
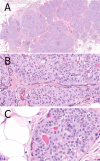
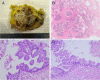
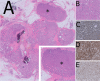
Papillary lesions of the breast represent a heterogeneous group of lesions including benign papillomas, papillomas with focal epithelial atypia, fully fledged ductal carcinoma in situ (DCIS) or lobular neoplasia, papillary DCIS, encapsulated papillary carcinomas without or with invasion, solid papillary carcinomas, and invasive papillary carcinomas. A micropapillary pattern characterized by lack of fibrous stalks within the papillae is observed in micropapillary DCIS and invasive micropapillary carcinoma. In addition, a variety of other rare breast lesions reveals a papillary architecture such as tall cell carcinoma with reversed polarity (TCCRP) and mucinous cystadenocarcinoma, adenomyoepithelioma, and secretory carcinoma. In addition, benign lesions such as usual ductal hyperplasia, apocrine metaplasia, gynecomastia, and juvenile papillomatosis may show a papillary or micropapillary architecture. Fragments of a benign papilloma in a breast biopsy are considered a lesion of uncertain malignant potential (B3 in the European classification) and excision is mostly recommended. Although the knowledge about molecular pathology of papillary breast lesions has increased, there is not sufficient evidence for diagnostically useful molecular features, yet. The aim of this review is to provide an update on papillary and micropapillary lesions with emphasis on problematic areas for daily diagnostic work including biopsies.
Keywords: Biopsy; Breast; DCIS; Ductal carcinoma in situ; Micropapillary; Papillary lesions.
© 2021. The Author(s).
Conflict of interest statement
Dr. Lax reports personal fees from Roche, AstraZeneca, Novartis, and Biogena outside the submitted work. All other authors declare no conflict of interest.
Figures











References
-
- Acs G, Khakpour N, Kiluk J, Lee MC, Laronga C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. Am J Surg Pathol. 2015;39:325–337. doi: 10.1097/PAS.0000000000000339. - DOI - PubMed
-
- Alsadoun N, MacGrogan G, Truntzer C, Lacroix-Triki M, Bedgedjian I, Koeb MH, El Alam E, Medioni D, Parent M, Wuithier P, Robert I, Boidot R, Arnould L. Solid papillary carcinoma with reverse polarity of the breast harbors specific morphologic, immunohistochemical and molecular profile in comparison with other benign or malignant papillary lesions of the breast: a comparative study of 9 additional cases. Mod Pathol. 2018;31:1367–1380. doi: 10.1038/s41379-018-0047-1. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

