Uptake and Survival Outcomes Following Immune Checkpoint Inhibitor Therapy Among Trial-Ineligible Patients With Advanced Solid Cancers
- PMID: 34734979
- PMCID: PMC8569600
- DOI: 10.1001/jamaoncol.2021.4971
Uptake and Survival Outcomes Following Immune Checkpoint Inhibitor Therapy Among Trial-Ineligible Patients With Advanced Solid Cancers
Abstract
Importance: Immune checkpoint inhibitors (ICIs) are part of standard of care for patients with many advanced solid tumors. Patients with poor performance status or organ dysfunction are traditionally ineligible to partake in pivotal randomized clinical trials of ICIs.
Objective: To assess ICI use and survival outcomes among patients with advanced cancers who are traditionally trial ineligible based on poor performance status or organ dysfunction.
Design, setting, and participants: This retrospective cohort study was conducted in 280 predominantly community oncology practices in the US and included 34 131 patients (9318 [27.3%] trial ineligible) who initiated first-line systemic therapy from January 2014 through December 2019 for newly diagnosed metastatic or recurrent nontargetable non-small cell lung, urothelial cell, renal cell, or hepatocellular carcinoma. Data analysis was performed from December 1, 2019, to June 1, 2021.
Exposures: Trial ineligibility (Eastern Cooperative Oncology Group performance status ≥2 or the presence of kidney or liver dysfunction); first-line systemic therapy.
Main outcomes and measures: The association between trial ineligibility and ICI monotherapy uptake was assessed using inverse probability-weighted (IPW) logistic regressions. The comparative survival outcomes following ICI and non-ICI therapy among trial-ineligible patients were assessed using treatment IPW survival analyses. Because we observed nonproportional hazards, we reported 12-month and 36-month restricted mean survival times (RMSTs) and time-varying hazard ratios (HRs) of less than 6 months and 6 months or greater.
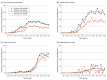
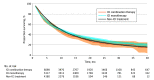
Results: Among the overall cohort (n = 34 131), the median (IQR) age was 70 (62-77) years; 23 586 (69%) were White individuals, and 14 478 (42%) were women. Over the study period, the proportion of patients receiving ICI monotherapy increased from 0% to 30.2% among trial-ineligible patients and 0.1% to 19.4% among trial-eligible patients. Trial ineligibility was associated with increased ICI monotherapy use (IPW-adjusted odds ratio compared with non-ICI therapy, 1.8; 95% CI, 1.7-1.9). Among trial-ineligible patients, there were no overall survival differences between ICI monotherapy, ICI combination therapy, and non-ICI therapy at 12 months (RMST, 7.8 vs 7.7 vs 8.1 months) or 36 months (RMST, 15.0 vs 13.9 vs 14.4 months). Compared with non-ICI therapy, ICI monotherapy showed evidence of early harm (IPW-adjusted HR within 6 months, 1.2; 95% CI, 1.1-1.2) but late benefit (adjusted HR among patients who survived 6 months, 0.8; 95% CI, 0.7-0.8).
Conclusions and relevance: In this cohort study, compared with trial-eligible patients, trial-ineligible patients with advanced cancers preferentially received first-line ICI therapy. A survival difference was not detected between ICI and non-ICI therapies among trial-ineligible patients. Positive results for ICI in phase 3 trials may not translate to this vulnerable population.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

