SARS-CoV-2 vaccine protection and deaths among US veterans during 2021
- PMID: 34735261
- PMCID: PMC9836205
- DOI: 10.1126/science.abm0620
SARS-CoV-2 vaccine protection and deaths among US veterans during 2021
Abstract
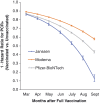
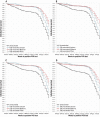
We report severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine effectiveness against infection (VE-I) and death (VE-D) by vaccine type in 780,225 veterans in the Veterans Health Administration, covering 2.7% of the US population. From February to October 2021, VE-I declined for all vaccine types, and the decline was greatest for the Janssen vaccine, resulting in a VE-I of 13.1%. Although breakthrough infection increased risk of death, vaccination remained protective against death in persons who became infected during the Delta variant surge. From July to October 2021, VE-D for age <65 years was 73.0% for Janssen, 81.5% for Moderna, and 84.3% for Pfizer-BioNTech; VE-D for age ≥65 years was 52.2% for Janssen, 75.5% for Moderna, and 70.1% for Pfizer-BioNTech. Findings support continued efforts to increase vaccination, booster campaigns, and multiple additional layers of protection against infection.
Figures

Comment in
References
-
- Pilishvili T., Fleming-Dutra K. E., Farrar J. L., Gierke R., Mohr N. M., Talan D. A., Krishnadasan A., Harland K. K., Smithline H. A., Hou P. C., Lee L. C., Lim S. C., Moran G. J., Krebs E., Steele M., Beiser D. G., Faine B., Haran J. P., Nandi U., Schrading W. A., Chinnock B., Henning D. J., LoVecchio F., Nadle J., Barter D., Brackney M., Britton A., Marceaux-Galli K., Lim S., Phipps E. C., Dumyati G., Pierce R., Markus T. M., Anderson D. J., Debes A. K., Lin M., Mayer J., Babcock H. M., Safdar N., Fischer M., Singleton R., Chea N., Magill S. S., Verani J., Schrag S., Yousaf A., Chung Y., Onukwube J., Xing W., Clinansmith B., Uribe L., Poronsky K. E., Hashimoto D. M., Bahamon M., St. Romain M., Kean E., Stubbs A., Roy S., Volturo G., Galbraith J., Crosby J. C., Fuentes M. R., Mulrow M., Lee J., Johnston H., Hansen A. J., Fridkin S. K., Wilson L. E., Lovett S., Christian M., Myers C., Ocampo V. L. S., Talbot K., Seidelman J., Milstone A. M., Hayden M., Samore M., Kwon J. H., Shirley D., Dillard D., Dobson J., Vaccine Effectiveness Among Healthcare Personnel Study Team , Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 U.S. sites, January–March 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 753–758 (2021). 10.15585/mmwr.mm7020e2 - DOI - PMC - PubMed
-
- Christie A., Henley S. J., Mattocks L., Fernando R., Lansky A., Ahmad F. B., Adjemian J., Anderson R. N., Binder A. M., Carey K., Dee D. L., Dias T., Duck W. M., Gaughan D. M., Lyons B. C., McNaghten A. D., Park M. M., Reses H., Rodgers L., Van Santen K., Walker D., Beach M. J., Decreases in COVID-19 Cases, Emergency department visits, hospital admissions, and deaths among older adults following the introduction of COVID-19 vaccine—United States, September 6, 2020–May 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 858–864 (2021). 10.15585/mmwr.mm7023e2 - DOI - PMC - PubMed
-
- Thompson M. G., Burgess J. L., Naleway A. L., Tyner H. L., Yoon S. K., Meece J., Olsho L. E. W., Caban-Martinez A. J., Fowlkes A., Lutrick K., Kuntz J. L., Dunnigan K., Odean M. J., Hegmann K. T., Stefanski E., Edwards L. J., Schaefer-Solle N., Grant L., Ellingson K., Groom H. C., Zunie T., Thiese M. S., Ivacic L., Wesley M. G., Lamberte J. M., Sun X., Smith M. E., Phillips A. L., Groover K. D., Yoo Y. M., Gerald J., Brown R. T., Herring M. K., Joseph G., Beitel S., Morrill T. C., Mak J., Rivers P., Harris K. M., Hunt D. R., Arvay M. L., Kutty P., Fry A. M., Gaglani M., Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—Eight U.S. locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 495–500 (2021). 10.15585/mmwr.mm7013e3 - DOI - PMC - PubMed
-
- Brown C. M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Sabo R. T., Hall N., Foreman A., Schubert P. L., Gallagher G. R., Fink T., Madoff L. C., Gabriel S. B., MacInnis B., Park D. J., Siddle K. J., Harik V., Arvidson D., Brock-Fisher T., Dunn M., Kearns A., Laney A. S., Outbreak of SARS-CoV-2 Infections, Including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1059–1062 (2021). 10.15585/mmwr.mm7031e2 - DOI - PMC - PubMed
-
- Herlihy R., Bamberg W., Burakoff A., Alden N., Severson R., Bush E., Kawasaki B., Berger B., Austin E., Shea M., Gabrieloff E., Matzinger S., Burdorf A., Nichols J., Goode K., Cilwick A., Stacy C., Staples E., Stringer G., Rapid increase in circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—Mesa County, Colorado, April-June 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1084–1087 (2021). 10.15585/mmwr.mm7032e2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

