Effects of a Partially Supervised Conditioning Program in Cystic Fibrosis: An International Multicenter, Randomized Controlled Trial (ACTIVATE-CF)
- PMID: 34735776
- PMCID: PMC8887001
- DOI: 10.1164/rccm.202106-1419OC
Effects of a Partially Supervised Conditioning Program in Cystic Fibrosis: An International Multicenter, Randomized Controlled Trial (ACTIVATE-CF)
Abstract
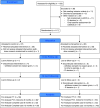
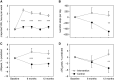
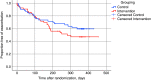
Rationale: The long-term effects of vigorous physical activity (PA) on lung function in cystic fibrosis are unclear. Objectives: To evaluate effects of a 12-month partially supervised PA intervention using motivational feedback. Methods: In a parallel-arm multicenter randomized controlled trial (ACTIVATE-CF), relatively inactive patients aged at least 12 years were randomly assigned (1:1 ratio) to an intervention group or control group. The intervention group consented to add 3 hours of vigorous PA per week, whereas the control group was asked not to change their PA behavior. Primary endpoint was change in percent predicted FEV1 (ΔFEV1) at 6 months. Secondary endpoints included PA, exercise capacity, exercise motives, time to first exacerbation and exacerbation rates, quality of life, anxiety, depression, stress, and blood glucose control. Data were analyzed using mixed linear models. Measurements and Main Results: A total of 117 patients (40% of target sample size) were randomized to an intervention (n = 60) or control group (n = 57). After 6 months, ΔFEV1 was significantly higher in the control group compared with the intervention group (2.70% predicted [95% confidence interval, 0.13-5.26]; P = 0.04). The intervention group reported increased vigorous PA compared with the control group at each study visit, had higher exercise capacity at 6 and 12 months, and higher PA at 12 months. No effects were seen in other secondary outcomes. Conclusions: ACTIVATE-CF increased vigorous PA and exercise capacity, with effects carried over for the subsequent 6 months, but resulted in better FEV1 in the control group.
Keywords: cystic fibrosis; exercise capacity; exercise program; physical activity; randomized controlled trial.
Figures

Comment in
-
Disconcerting and Counterintuitive Findings from a Trial of Exercise in Cystic Fibrosis: Can Exercise Make Our Patients Worse?Am J Respir Crit Care Med. 2022 Feb 1;205(3):269-270. doi: 10.1164/rccm.202110-2446ED. Am J Respir Crit Care Med. 2022. PMID: 34856109 Free PMC article. No abstract available.
References
-
- Barker M, Hebestreit A, Gruber W, Hebestreit H. Exercise testing and training in German CF centers. Pediatr Pulmonol . 2004;37:351–355. - PubMed
-
- Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol . 2002;33:194–200. - PubMed
-
- Kriemler S, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Effect of supervised training on FEV1 in cystic fibrosis: a randomised controlled trial. J Cyst Fibros . 2013;12:714–720. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

