Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine
- PMID: 34735795
- PMCID: PMC8519781
- DOI: 10.1016/j.cell.2021.10.011
Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine
Abstract
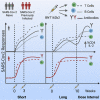
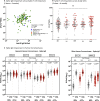
Extension of the interval between vaccine doses for the BNT162b2 mRNA vaccine was introduced in the United Kingdom to accelerate population coverage with a single dose. At this time, trial data were lacking, and we addressed this in a study of United Kingdom healthcare workers. The first vaccine dose induced protection from infection from the circulating alpha (B.1.1.7) variant over several weeks. In a substudy of 589 individuals, we show that this single dose induces severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody (NAb) responses and a sustained B and T cell response to the spike protein. NAb levels were higher after the extended dosing interval (6-14 weeks) compared with the conventional 3- to 4-week regimen, accompanied by enrichment of CD4+ T cells expressing interleukin-2 (IL-2). Prior SARS-CoV-2 infection amplified and accelerated the response. These data on dynamic cellular and humoral responses indicate that extension of the dosing interval is an effective immunogenic protocol.
Keywords: B cell; BNT162b2; COVID-19; SARS-CoV-2; T cell; antibody; dosing interval; neutralization; vaccine; variants of concern.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.J.P. is Chair of the United Kingdom Department of Health and Social Care (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in policy decisions on COVID-19 vaccines. He is a member of the WHO’s SAGE. The views expressed in this article do not necessarily represent the views of the DHSC, JCVI, or WHO. A.J.P. is chief investigator on clinical trials of Oxford University’s COVID-19 vaccine funded by NIHR. Oxford University has entered a joint COVID-19 vaccine development partnership with AstraZeneca.
Figures








References
-
- Amirthalingam G., Bernal J.L., Andrews N.J., Whitaker H., Gower C., Stowe J., Tessier E., Subbarao V., Ireland G., Baawuah F., et al. Higher serological responses and increased vaccine effectiveness demonstrate the value of extended vaccine schedules in combatting COVID-19 in England. medRxiv. 2021 doi: 10.1101/2021.07.26.21261140. - DOI
-
- Angyal A., Longet S., Moore S., Payne R., Harding A., Tipton T., Rongkard P., Ali M., Hering L., Meardon N., et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Lancet Microbe in press. 2021 doi: 10.2139/ssrn.3812375. Preprint published online March 25, 2021. - DOI - PMC - PubMed
-
- Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., et al. Oxford COVID Vaccine Trial Group Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. - PubMed
-
- Betts M.R., Brenchley J.M., Price D.A., De Rosa S.C., Douek D.C., Roederer M., Koup R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

