Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat
- PMID: 34736493
- PMCID: PMC8567657
- DOI: 10.1186/s12974-021-02303-y
Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat
Abstract
Background: Chronic unpredictable mild stress (CUMS) can not only lead to depression-like behavior but also change the composition of the gut microbiome. Regulating the gut microbiome can have an antidepressant effect, but the mechanism by which it improves depressive symptoms is not clear. Short-chain fatty acids (SCFAs) are small molecular compounds produced by the fermentation of non-digestible carbohydrates. SFCAs are ubiquitous in intestinal endocrine and immune cells, making them important mediators of gut microbiome-regulated body functions. The balance between the pro- and anti-inflammatory microglia plays an important role in the occurrence and treatment of depression caused by chronic stress. Non-absorbable antibiotic rifaximin can regulate the structure of the gut microbiome. We hypothesized that rifaximin protects against stress-induced inflammation and depression-like behaviors by regulating the abundance of fecal microbial metabolites and the microglial functions.
Methods: We administered 150 mg/kg rifaximin intragastrically to rats exposed to CUMS for 4 weeks and investigated the composition of the fecal microbiome, the content of short-chain fatty acids in the serum and brain, the functional profiles of microglia and hippocampal neurogenesis.
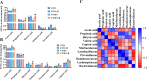
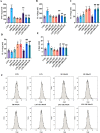
Results: Our results show that rifaximin ameliorated depressive-like behavior induced by CUMS, as reflected by sucrose preference, the open field test and the Morris water maze. Rifaximin increased the relative abundance of Ruminococcaceae and Lachnospiraceae, which were significantly positively correlated with the high level of butyrate in the brain. Rifaximin increased the content of anti-inflammatory factors released by microglia, and prevented the neurogenic abnormalities caused by CUMS.
Conclusions: These results suggest that rifaximin can regulate the inflammatory function of microglia and play a protective role in pubertal neurodevelopment during CUMS by regulating the gut microbiome and short-chain fatty acids.
Keywords: Adolescence; Chronic unpredictable mild stress (CUMS); Gut microbiome; Microglia; Neurogenesis; Rifaximin; Short chain fatty acid (SCFA).
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

