Interplay between intraocular and intracranial pressure effects on the optic nerve head in vivo
- PMID: 34736887
- PMCID: PMC8665145
- DOI: 10.1016/j.exer.2021.108809
Interplay between intraocular and intracranial pressure effects on the optic nerve head in vivo
Abstract
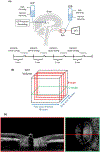
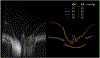
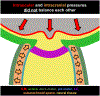
Intracranial pressure (ICP) has been proposed to play an important role in the sensitivity to intraocular pressure (IOP) and susceptibility to glaucoma. However, the in vivo effects of simultaneous, controlled, acute variations in ICP and IOP have not been directly measured. We quantified the deformations of the anterior lamina cribrosa (ALC) and scleral canal at Bruch's membrane opening (BMO) under acute elevation of IOP and/or ICP. Four eyes of three adult monkeys were imaged in vivo with OCT under four pressure conditions: IOP and ICP either at baseline or elevated. The BMO and ALC were reconstructed from manual delineations. From these, we determined canal area at the BMO (BMO area), BMO aspect ratio and planarity, and ALC median depth relative to the BMO plane. To better account for the pressure effects on the imaging, we also measured ALC visibility as a percent of the BMO area. Further, ALC depths were analyzed only in regions where the ALC was visible in all pressure conditions. Bootstrap sampling was used to obtain mean estimates and confidence intervals, which were then used to test for significant effects of IOP and ICP, independently and in interaction. Response to pressure manipulation was highly individualized between eyes, with significant changes detected in a majority of the parameters. Significant interactions between ICP and IOP occurred in all measures, except ALC visibility. On average, ICP elevation expanded BMO area by 0.17 mm2 at baseline IOP, and contracted BMO area by 0.02 mm2 at high IOP. ICP elevation decreased ALC depth by 10 μm at baseline IOP, but increased depth by 7 μm at high IOP. ALC visibility decreased as ICP increased, both at baseline (-10%) and high IOP (-17%). IOP elevation expanded BMO area by 0.04 mm2 at baseline ICP, and contracted BMO area by 0.09 mm2 at high ICP. On average, IOP elevation caused the ALC to displace 3.3 μm anteriorly at baseline ICP, and 22 μm posteriorly at high ICP. ALC visibility improved as IOP increased, both at baseline (5%) and high ICP (8%). In summary, changing IOP or ICP significantly deformed both the scleral canal and the lamina of the monkey ONH, regardless of the other pressure level. There were significant interactions between the effects of IOP and those of ICP on LC depth, BMO area, aspect ratio and planarity. On most eyes, elevating both pressures by the same amount did not cancel out the effects. Altogether our results show that ICP affects sensitivity to IOP, and thus that it can potentially also affect susceptibility to glaucoma.
Keywords: Biomechanics; Glaucoma; Intracranial pressure; Intraocular pressure; Lamina cribrosa; OCT; Optic nerve head; Translaminar pressure.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures




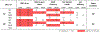
References
-
- Furlanetto RL, Park SC, Damle UJ, et al. Posterior displacement of the lamina cribrosa in glaucoma: in vivo interindividual and intereye comparisons. Invest Ophthalmol Vis Sci. 2013;54(7):4836–4842. - PubMed
-
- Quigley HA, Sanchez RM, Dunkelberger GR, L’Hernault NL, Baginski TA. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28(6):913–920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

