Serum integrative omics reveals the landscape of human diabetic kidney disease
- PMID: 34737094
- PMCID: PMC8609166
- DOI: 10.1016/j.molmet.2021.101367
Serum integrative omics reveals the landscape of human diabetic kidney disease
Abstract
Objective: Diabetic kidney disease (DKD) is the most common microvascular complication of type 2 diabetes mellitus (2-DM). Currently, urine and kidney biopsy specimens are the major clinical resources for DKD diagnosis. Our study proposes to evaluate the diagnostic value of blood in monitoring the onset of DKD and distinguishing its status in the clinic.
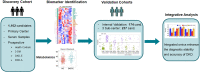
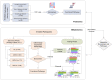
Methods: This study recruited 1,513 participants including healthy adults and patients diagnosed with 2-DM, early-stage DKD (DKD-E), and advanced-stage DKD (DKD-A) from 4 independent medical centers. One discovery and four testing cohorts were established. Sera were collected and subjected to training proteomics and large-scale metabolomics.
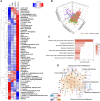
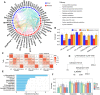
Results: Deep profiling of serum proteomes and metabolomes revealed several insights. First, the training proteomics revealed that the combination of α2-macroglobulin, cathepsin D, and CD324 could serve as a surrogate protein biomarker for monitoring DKD progression. Second, metabolomics demonstrated that galactose metabolism and glycerolipid metabolism are the major disturbed metabolic pathways in DKD, and serum metabolite glycerol-3-galactoside could be used as an independent marker to predict DKD. Third, integrating proteomics and metabolomics increased the diagnostic and predictive stability and accuracy for distinguishing DKD status.
Conclusions: Serum integrative omics provide stable and accurate biomarkers for early warning and diagnosis of DKD. Our study provides a rich and open-access data resource for optimizing DKD management.
Keywords: Diabetic kidney disease; Machine learning; Metabolomics; Proteomics; Serum; Type 2 diabetes mellitus.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Research and Clinical Practice. 2019;157:107843. 9(th) edition. - PubMed
-
- Atkins R.C., Zimmet P., Committee I-IWKDS Diabetes: diabetic kidney disease: act now or pay later. Nature Reviews Nephrology. 2010;6(3):134–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

