Glutamine Imaging: A New Avenue for Glioma Management
- PMID: 34737183
- PMCID: PMC8757564
- DOI: 10.3174/ajnr.A7333
Glutamine Imaging: A New Avenue for Glioma Management
Abstract
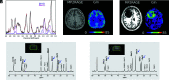
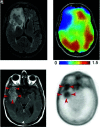
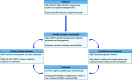
The glutamine pathway is emerging as an important marker of cancer prognosis and a target for new treatments. In gliomas, the most common type of brain tumors, metabolic reprogramming leads to abnormal consumption of glutamine as an energy source, and increased glutamine concentrations are associated with treatment resistance and proliferation. A key challenge in the development of glutamine-based biomarkers and therapies is the limited number of in vivo tools to noninvasively assess local glutamine metabolism and monitor its changes. In this review, we describe the importance of glutamine metabolism in gliomas and review the current landscape of translational and emerging imaging techniques to measure glutamine in the brain. These techniques include MRS, PET, SPECT, and preclinical methods such as fluorescence and mass spectrometry imaging. Finally, we discuss the roadblocks that must be overcome before incorporating glutamine into a personalized approach for glioma management.
© 2022 by American Journal of Neuroradiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
