Plasma proteins elevated in severe asthma despite oral steroid use and unrelated to Type-2 inflammation
- PMID: 34737220
- PMCID: PMC8850689
- DOI: 10.1183/13993003.00142-2021
Plasma proteins elevated in severe asthma despite oral steroid use and unrelated to Type-2 inflammation
Abstract
Rationale: Asthma phenotyping requires novel biomarker discovery.
Objectives: To identify plasma biomarkers associated with asthma phenotypes by application of a new proteomic panel to samples from two well-characterised cohorts of severe (SA) and mild-to-moderate (MMA) asthmatics, COPD subjects and healthy controls (HCs).
Methods: An antibody-based array targeting 177 proteins predominantly involved in pathways relevant to inflammation, lipid metabolism, signal transduction and extracellular matrix was applied to plasma from 525 asthmatics and HCs in the U-BIOPRED cohort, and 142 subjects with asthma and COPD from the validation cohort BIOAIR. Effects of oral corticosteroids (OCS) were determined by a 2-week, placebo-controlled OCS trial in BIOAIR, and confirmed by relation to objective OCS measures in U-BIOPRED.
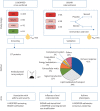
Results: In U-BIOPRED, 110 proteins were significantly different, mostly elevated, in SA compared to MMA and HCs. 10 proteins were elevated in SA versus MMA in both U-BIOPRED and BIOAIR (alpha-1-antichymotrypsin, apolipoprotein-E, complement component 9, complement factor I, macrophage inflammatory protein-3, interleukin-6, sphingomyelin phosphodiesterase 3, TNF receptor superfamily member 11a, transforming growth factor-β and glutathione S-transferase). OCS treatment decreased most proteins, yet differences between SA and MMA remained following correction for OCS use. Consensus clustering of U-BIOPRED protein data yielded six clusters associated with asthma control, quality of life, blood neutrophils, high-sensitivity C-reactive protein and body mass index, but not Type-2 inflammatory biomarkers. The mast cell specific enzyme carboxypeptidase A3 was one major contributor to cluster differentiation.
Conclusions: The plasma proteomic panel revealed previously unexplored yet potentially useful Type-2-independent biomarkers and validated several proteins with established involvement in the pathophysiology of SA.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: M. Sparreman Mikus has nothing to disclose. Conflict of interest: J. Kolmert reports personal fees from Gesynta Pharma AB. Conflict of interest: L.I. Andersson has nothing to disclose. Conflict of interest: J. Östling has nothing to disclose. Conflict of interest: R.G. Knowles has nothing to disclose. Conflict of interest: C. Gómez has nothing to disclose. Conflict of interest: M. Ericsson has nothing to disclose. Conflict of interest: J-O. Thörngren has nothing to disclose. Conflict of interest: P. Emami Khoonsari has nothing to disclose. Conflict of interest: B. Dahlén reports personal fees from AstraZeneca, Teva, Sanofi and grants from Novartis and GlaxoSmithKline outside the submitted work. Conflict of interest: M. Kupczyk has nothing to disclose. Conflict of interest: B. De Meulder report grants from the Innovative Medicines Initiative during the conduct of the study. Conflict of interest: C. Auffray report grants from the Innovative Medicines Initiative during the conduct of the study. Conflict of interest: P.S. Bakke reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim and Chiesi outside the submitted work. Conflict of interest: B. Beghe reports personal fees from AstraZeneca, Boehringer Ingelheim, Menarini and GlaxoSmithKline outside the submitted work. Conflict of interest: E.H. Bel reports grants and personal fees from GlaxoSmithKline, AstraZeneca, Novartis, TEVA, Sanofi/Regeneron, Chiesi, and Sterna outside the submitted work. Conflict of interest: M. Caruso has nothing to disclose. Conflict of interest: P. Chanez has nothing to disclose. Conflict of interest: B. Chawes has nothing to disclose. Conflict of interest: S.J. Fowler has nothing to disclose. Conflict of interest: M. Gaga reports grants and personal fees from Novartis, Menarini, Merck Sharp & Dohme, BMS, Galapagos, and AstraZeneca outside the submitted work. Conflict of interest: T. Geiser has nothing to disclose. Conflict of interest: M. Gjomarkaj has nothing to disclose. Conflict of interest: I. Horváth reports grants from EFPIA during the conduct of the study, and personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Boehringer-Ingelheim, Sandoz, Teva and Chiesi outside the submitted work. Conflict of interest: P.H. Howarth has nothing to disclose. Conflict of interest: S.L. Johnston reports personal fees from Virtus Respiratory Research, Myelo Therapeutics GmbH, Concert Pharmaceuticals, Bayer, Synairgen, Novartis, Boehringer Ingelheim, Chiesi, Gerson Lehrman Group, resTORbio, Bioforce, Materia Medical Holdings, PrepBio Pharma, Pulmotect, Virion Health, Lallemand Pharma and AstraZeneca outside the submitted work. In addition, Sebastian L. Johnston also has three patents (Anti-virus therapy for respiratory diseases, UK patent application No. GB 0405634.7, Interferon-Beta for Anti-Virus Therapy for Respiratory Diseases, International Patent Application No. PCT/ GB05/50031 and Interferon Lambda therapy for the treatment of respiratory disease, UK patent application No.6779645.9). Conflict of interest: G. Joos reports grants and personal fees from AstraZeneca, Bayer, Chiesi, Eureca vzw, GlaxoSmithKline and Teva outside the submitted work. Conflict of interest: N. Krug has nothing to disclose. Conflict of interest: P. Montuschi has nothing to disclose. Conflict of interest: J. Musial has nothing to disclose. Conflict of interest: E. Niżankowska-Mogilnicka has nothing to disclose. Conflict of interest: H.K. Olsson reports other support from AstraZeneca outside the submitted work. Conflict of interest: A. Papi reports grants, personal fees, non-financial support and others from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Chiesi, Teva, Mundipharma, Zambon, Novartis, Menarini, Sanofi/Regeneron, Roche, Fondazione Salvatore Maugeri, Chiesi and Edmond pharma outside the submitted work. Conflict of interest: K.F. Rabe reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Sanofi Aventis, MERCK SHARP & DOHME, Novartis, Orion Cooperation, Berlin Chemie, Roche, Chiesi and grants for research from the Ministry of Education and Science, Germany. Conflict of interest: T. Sandström has nothing to disclose. Conflict of interest: D.E. Shaw reports personal fees and non-financial support from GlaxoSmithKline, Novartis and AstraZeneca outside the submitted work. Conflict of interest: N.M. Siafakas has nothing to disclose. Conflict of interest: M. Uhlen has nothing to disclose. Conflict of interest: J.H. Riley is an employee and shareholder in GlaxoSmithKline. Conflict of interest: S. Bates is an employee and shareholder in GlaxoSmithKline. Conflict of interest: R.J.M. Middelveld reports grants from the Swedish Strategic Research Foundation, AstraZeneca, the Swedish Heart Lung Foundation and the Swedish Asthma and Allergy Association outside the submitted work. Conflict of interest: C.E. Wheelock has nothing to disclose. Conflict of interest: K.F. Chung reports grants and personal fees from GlaxoSmithKline, AstraZeneca, Novartis, Merck, Boehringer Ingelheim, Roche and Shionogi outside the submitted work. Conflict of interest: I.M. Adcock has nothing to disclose. Conflict of interest: P.J. Sterk reports grants to Amsterdam UMC from the public private Innovative Medicines Initiative (IMI) covered by the European Union (EU) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) during the conduct of the study. Conflict of interest: R. Djukanovic reports receiving fees for lectures at symposia organised by Novartis, GlaxoSmithKline, AstraZeneca and Teva, consultation fees from Teva and Novartis; he is a co-founder and current consultant and has shares in Synairgen. Conflict of interest: P. Nilsson has nothing to disclose. Conflict of interest: S-E. Dahlén reports personal fees from AstraZeneca, Cayman Chemicals, GlaxoSmithKline, Novartis, Regeneron, Sanofi and Teva outside the submitted work. Conflict of interest: A. James has nothing to disclose.
Figures


Comment in
-
The quest for biomarkers in asthma: challenging the T2 versus non-T2 paradigm.Eur Respir J. 2022 Feb 17;59(2):2102669. doi: 10.1183/13993003.02669-2021. Print 2022 Feb. Eur Respir J. 2022. PMID: 35177484 No abstract available.
-
Reply: U-BIOPRED/BIOAIR proteins: inflammation or infection?Eur Respir J. 2022 Dec 1;60(6):2201795. doi: 10.1183/13993003.01795-2022. Print 2022 Dec. Eur Respir J. 2022. PMID: 36202410 Free PMC article.
-
U-BIOPRED/BIOAIR proteins: inflammation or infection?Eur Respir J. 2022 Dec 1;60(6):2200571. doi: 10.1183/13993003.00571-2022. Print 2022 Dec. Eur Respir J. 2022. PMID: 36202417 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
