External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry
- PMID: 34737227
- PMCID: PMC9245192
- DOI: 10.1183/13993003.02419-2021
External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry
Abstract
Introduction: Contemporary risk assessment tools categorise patients with pulmonary arterial hypertension (PAH) as low, intermediate or high risk. A minority of patients achieve low risk status with most remaining intermediate risk. Our aim was to validate a four-stratum risk assessment approach categorising patients as low, intermediate-low, intermediate-high or high risk, as proposed by the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) investigators.
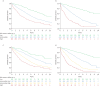
Methods: We evaluated incident patients from the French PAH Registry and applied a four-stratum risk method at baseline and at first reassessment. We applied refined cut-points for three variables: World Health Organization functional class, 6-min walk distance and N-terminal pro-brain natriuretic peptide. We used Kaplan-Meier survival analyses and Cox proportional hazards regression to assess survival according to three-stratum and four-stratum risk approaches.
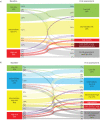
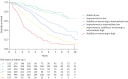
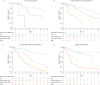
Results: At baseline (n=2879), the four-stratum approach identified four distinct risk groups and performed slightly better than a three-stratum method for predicting mortality. Four-stratum model discrimination was significantly higher than the three-stratum method when applied during follow-up and refined risk categories among subgroups with idiopathic PAH, connective tissue disease-associated PAH, congenital heart disease and portopulmonary hypertension. Using the four-stratum approach, 53% of patients changed risk category from baseline compared to 39% of patients when applying the three-stratum approach. Those who achieved or maintained a low risk status had the best survival, whereas there were more nuanced differences in survival for patients who were intermediate-low and intermediate-high risk.
Conclusions: The four-stratum risk assessment method refined risk prediction, especially within the intermediate risk category of patients, performed better at predicting survival and was more sensitive to change than the three-stratum approach.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: A. Boucly reports personal fees from Actelion, Bayer and Merck, outside the submitted work. Conflict of interest: J. Weatherald reports grants, personal fees and non-financial support from Janssen Inc., grants, personal fees and non-financial support from Actelion, personal fees and non-financial support from Bayer, personal fees from Novartis, outside the submitted work. Conflict of interest: L. Savale reports personal fees from Actelion, personal fees from MSD, grants and personal fees from GSK, outside the submitted work. Conflict of interest: P. de Groote reports consulting fees from Actelion, Janssen, MSD, Novartis, Servier, Boehringer Ingelheim, Abbott, Boston, AstraZeneca, Bayer; lecture honoraria from Abbott, Vifor, MSD, Servier, Novartis, AstraZeneca, Actelion, Janssen, Medtronic; outside the submitted work. Conflict of interest: V. Cottin reports advisory board fees and non-financial support from Actelion, advisory board fees from Bayer/MSD, outside the submitted work. Conflict of interest: G. Prévot reports personal fees from Actelion and GSK, outside the submitted work. Conflict of interest: A. Chaouat reports consulting fees from GSK, Actelion and Bayer, outside the submitted work. Conflict of interest: F. Picard has nothing to disclose. Conflict of interest: D. Horeau-Langlard reports grants from Acceleron, outside the submitted work. Conflict of interest: A. Bourdin reports grants from AstraZeneca and Boehringer Ingelheim; consulting fees from AstraZeneca, GSK, Novartis, Boehringer Ingelheim, Chiesi, Sanofi Regeneron, Amgen; lecture honoraria from AstraZeneca, GSK, Novartis, Boehringer Ingelheim, Chiesi, Sanofi Regeneron, Roche; travel support from Boehringer Ingelheim, Chiesi, Sanofi Regeneron, AstraZeneca, GSK, Roche; participation on advisory boards at AB science, AstraZeneca, GSK, Sanofi Regeneron, Novartis, Acceleron; and acted as investigator in clinical trials for Vertex, Abbvie, Galapagos, Fibrogen, Nuvaira, PulmonX, Gossamer, Acceleron; outside the submitted work. Conflict of interest: E-M. Jutant has nothing to disclose. Conflict of interest: A. Beurnier has nothing to disclose. Conflict of interest: M. Jevnikar has nothing to disclose. Conflict of interest: X. Jais reports grants from Bayer, Janssen and Merck; lecture honoraria from Janssen and Merck; outside the submitted work. Conflict of interest: G. Simonneau reports grants and personal fees from Janssen (formerly Actelion), Bayer and MSD; personal fees from Acceleron, outside the submitted work. Conflict of interest: D. Montani reports grants from Acceleron, Janssen and Merck; steering committee fees from Acceleron; lecture honoraria from Bayer, Janssen and Merck; outside the submitted work. Conflict of interest: O. Sitbon reports grants from Acceleron, Janssen, GSK and MSD; steering committee fees from Gossamer Bio, Janssen and MSD; lecture honoraria from AOP Orphan, Janssen, Ferrer and MSD; advisory board participation at Acceleron, Janssen and MSD; outside the submitted work. Conflict of interest: M. Humbert reports grants, steering committee consulting fees, and advisory board participation from Acceleron, Janssen and Merck; lecture honoraria from AOP, Janssen and Merck; steering committee participation at United Therapeutics; outside the submitted work.
Figures
Comment in
-
Refined risk stratification in pulmonary arterial hypertension and timing of lung transplantation.Eur Respir J. 2022 Aug 4;60(2):2103087. doi: 10.1183/13993003.03087-2021. Print 2022 Aug. Eur Respir J. 2022. PMID: 35144993 No abstract available.
References
-
- Galiè N, Humbert M, Vachiery J-L, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. doi:10.1183/13993003.01032-2015 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
