Distinct SARS-CoV-2 antibody reactivity patterns elicited by natural infection and mRNA vaccination
- PMID: 34737318
- PMCID: PMC8568980
- DOI: 10.1038/s41541-021-00396-3
Distinct SARS-CoV-2 antibody reactivity patterns elicited by natural infection and mRNA vaccination
Abstract
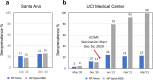
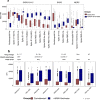
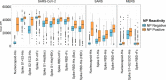
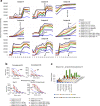
We analyzed data from two ongoing COVID-19 longitudinal serological surveys in Orange County, CA., between April 2020 and March 2021. A total of 8476 finger stick blood specimens were collected before and after a vaccination campaign. IgG levels were determined using a multiplex antigen microarray containing antigens from SARS-CoV-2, SARS, MERS, Common CoV, and Influenza. Twenty-six percent of specimens from unvaccinated Orange County residents in December 2020 were SARS-CoV-2 seropositive; out of 852 seropositive individuals 77 had symptoms and 9 sought medical care. The antibody response was predominantly against nucleocapsid (NP), full length, and S2 domain of spike. Anti-receptor binding domain (RBD) reactivity was low and not cross-reactive against SARS S1 or SARS RBD. A vaccination campaign at the University of California Irvine Medical Center (UCIMC) started on December, 2020 and 6724 healthcare workers were vaccinated within 3 weeks. Seroprevalence increased from 13% pre-vaccination to 79% post-vaccination in January, 93% in February, and 99% in March. mRNA vaccination induced higher antibody levels than natural exposure, especially against the RBD domain and cross-reactivity against SARS RBD and S1 was observed. Nucleocapsid protein antibodies can be used to distinguish vaccinees to classify pre-exposure to SARS-CoV-2 Previously infected individuals developed higher antibody titers to the vaccine than non pre-exposed individuals. Hospitalized patients in intensive care with severe disease reach significantly higher antibody levels than mild cases, but lower antibody levels compared to the vaccine. These results indicate that mRNA vaccination rapidly induces a much stronger and broader antibody response than SARS-CoV-2 infection.
© 2021. The Author(s).
Conflict of interest statement
The coronavirus antigen microarray is intellectual property of the Regents of the University of California that is licensed for commercialization to Nanommune Inc. (Irvine, CA), a private company for which Philip L. Felgner is the largest shareholder and several co-authors (de Assis, Jain, Nakajima, Jasinskas, Davies, and Khan) also own shares. Nanommune Inc. has a business partnership with Sino Biological Inc. (Beijing, China) which expressed and purified the antigens used in this study. The other authors have no competing interests.
Figures



Similar articles
-
A Serological Analysis of the Humoral Immune Responses of Anti-RBD IgG, Anti-S1 IgG, and Anti-S2 IgG Levels Correlated to Anti-N IgG Positivity and Negativity in Sicilian Healthcare Workers (HCWs) with Third Doses of the mRNA-Based SARS-CoV-2 Vaccine: A Retrospective Cohort Study.Vaccines (Basel). 2023 Jun 23;11(7):1136. doi: 10.3390/vaccines11071136. Vaccines (Basel). 2023. PMID: 37514952 Free PMC article.
-
Machine Learning Classification of Time since BNT162b2 COVID-19 Vaccination Based on Array-Measured Antibody Activity.Life (Basel). 2023 May 31;13(6):1304. doi: 10.3390/life13061304. Life (Basel). 2023. PMID: 37374086 Free PMC article.
-
Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients.BMC Med. 2021 Aug 23;19(1):208. doi: 10.1186/s12916-021-02090-6. BMC Med. 2021. PMID: 34420521 Free PMC article.
-
Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers.Vaccines (Basel). 2022 Jul 22;10(8):1169. doi: 10.3390/vaccines10081169. Vaccines (Basel). 2022. PMID: 35893818 Free PMC article.
-
Low SARS-CoV-2 infection rates and high vaccine-induced immunity among German healthcare workers at the end of the third wave of the COVID-19 pandemic.Int J Hyg Environ Health. 2021 Sep;238:113851. doi: 10.1016/j.ijheh.2021.113851. Epub 2021 Sep 25. Int J Hyg Environ Health. 2021. PMID: 34601375 Free PMC article.
Cited by
-
Neutralizing antibodies against SARS-CoV-2 are higher but decline faster in mRNA vaccinees compared to individuals with natural infection.J Travel Med. 2022 Dec 27;29(8):taac130. doi: 10.1093/jtm/taac130. J Travel Med. 2022. PMID: 36342115 Free PMC article.
-
Longitudinal study comparing IgG antibodies induced by heterologous prime-boost COVID-19 vaccine.J Med Virol. 2023 Jan;95(1):e28379. doi: 10.1002/jmv.28379. J Med Virol. 2023. PMID: 36478244 Free PMC article.
-
The rapid progress in COVID vaccine development and implementation.NPJ Vaccines. 2022 Feb 10;7(1):20. doi: 10.1038/s41541-022-00442-8. NPJ Vaccines. 2022. PMID: 35145102 Free PMC article. No abstract available.
-
mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern.Immunity. 2022 Feb 8;55(2):355-365.e4. doi: 10.1016/j.immuni.2022.01.001. Epub 2022 Jan 6. Immunity. 2022. PMID: 35090580 Free PMC article.
-
Identification of undetected SARS-CoV-2 infections by clustering of Nucleocapsid antibody trajectories.Nat Commun. 2025 May 14;16(1):4466. doi: 10.1038/s41467-025-57370-z. Nat Commun. 2025. PMID: 40368905 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

