Treatment With Melatonin After Corneal Graft Attenuates Rejection
- PMID: 34737710
- PMCID: PMC8560893
- DOI: 10.3389/fphar.2021.778892
Treatment With Melatonin After Corneal Graft Attenuates Rejection
Abstract
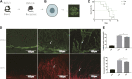
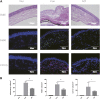
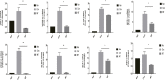
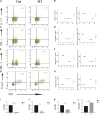
Background: Immunologic graft rejection is the main complication of corneal transplants. This study aimed to investigate the effect of melatonin (MT) on the rejection of corneal transplantation. Methods: Corneal allografts were performed by grafting corneas from BALB/C mice to C57BL/6 hosts. MT (50 mg/kg) was intraperitoneally injected into the hosts every day from the day of transplantation. The survival of grafts was observed by slit lamp biomicroscopy, and inflammatory cell infiltration was detected by hematoxylin and eosin staining and immunohistochemistry. The balance of Teff and Treg immune cells in draining lymph nodes (DLNs) was detected by flow cytometry. The levels of cytokines related to the grafts and DLNs were detected using real-time fluorescence quantitative PCR. Additionally, we used the mouse macrophage line RAW264.7 to study the effect of MT on the activation of NLRP3 inflammatory body. Results: MT treatment improved the graft survival rate, reduced inflammatory cell infiltration in the graft, decreased the percentage of Th1/Th17 cells in the DLNs, and increased the percentage of Treg cells. Melatonin inhibited the activation of the NLRP3 inflammasome, thereby reducing the expression of IL-1β and other related proinflammatory cytokines such as MCP-1, MIP-1, NLRP3, ASC, TNF-a and VEGF-A (all p < 0.05). Conclusion: Our study demonstrates that MT promotes the survival of mouse corneal grafts by inhibiting NLRP3-mediated immune regulation, reducing immune cell activation and cell migration, and inhibiting the production of inflammatory-related cytokines. Treatment with MT might provide a potential clinical therapeutic target for corneal transplantation.
Keywords: CD4+ T cells; NLRP3 inflammasome; corneal transplant; macrophages; melatonin.
Copyright © 2021 Zhu, Peng, Shen, Zhong, Song, Wang and Ling.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation with the authors at the time of the review.
Figures

Similar articles
-
Resolvin E1 Inhibits Corneal Allograft Rejection in High-Risk Corneal Transplantation.Invest Ophthalmol Vis Sci. 2018 Aug 1;59(10):3911-3919. doi: 10.1167/iovs.18-24562. Invest Ophthalmol Vis Sci. 2018. PMID: 30073362
-
Blockage of P2X7R suppresses Th1/Th17-mediated immune responses and corneal allograft rejection via inhibiting NLRP3 inflammasome activation.Exp Eye Res. 2021 Nov;212:108792. doi: 10.1016/j.exer.2021.108792. Epub 2021 Oct 14. Exp Eye Res. 2021. PMID: 34656546
-
Local Delivery of Regulatory T Cells Promotes Corneal Allograft Survival.Transplantation. 2019 Jan;103(1):182-190. doi: 10.1097/TP.0000000000002442. Transplantation. 2019. PMID: 30247445 Free PMC article.
-
Endogenous Toll-like Receptor 2 Modulates Th1/Treg-Promoting Dendritic Cells in Mice Corneal Transplantation Model.Curr Eye Res. 2020 Jul;45(7):774-781. doi: 10.1080/02713683.2019.1705491. Epub 2019 Dec 26. Curr Eye Res. 2020. PMID: 31842628
-
Neutralization of mouse interleukin-17 bioactivity inhibits corneal allograft rejection.Mol Vis. 2011;17:2148-56. Epub 2011 Aug 11. Mol Vis. 2011. PMID: 21850190 Free PMC article.
Cited by
-
A Selective Melatonin 2 Receptor Agonist, IIK7, Relieves Blue Light-Induced Corneal Damage by Modulating the Process of Autophagy and Apoptosis.Int J Mol Sci. 2024 Oct 19;25(20):11243. doi: 10.3390/ijms252011243. Int J Mol Sci. 2024. PMID: 39457025 Free PMC article.
-
The Therapeutic Trip of Melatonin Eye Drops: From the Ocular Surface to the Retina.Pharmaceuticals (Basel). 2024 Mar 29;17(4):441. doi: 10.3390/ph17040441. Pharmaceuticals (Basel). 2024. PMID: 38675402 Free PMC article. Review.
-
The NLRP3 Inflammasome Is Required for Protection Against Pseudomonas Keratitis.Invest Ophthalmol Vis Sci. 2023 Feb 1;64(2):11. doi: 10.1167/iovs.64.2.11. Invest Ophthalmol Vis Sci. 2023. PMID: 36749596 Free PMC article.
-
The Role and Therapeutic Potential of Melatonin in Degenerative Fundus Diseases: Diabetes Retinopathy and Age-Related Macular Degeneration.Drug Des Devel Ther. 2024 Jun 18;18:2329-2346. doi: 10.2147/DDDT.S471525. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38911030 Free PMC article. Review.
-
Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway.J Nanobiotechnology. 2023 Sep 16;21(1):332. doi: 10.1186/s12951-023-02087-8. J Nanobiotechnology. 2023. PMID: 37716974 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

