To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1
- PMID: 34737737
- PMCID: PMC8560739
- DOI: 10.3389/fimmu.2021.708227
To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1
Abstract
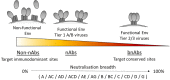
Since their discovery, antibodies capable of broad neutralisation have been at the forefront of HIV-1 research and are of particular interest due to in vivo passive transfer studies demonstrating their potential to provide protection. Currently an exact definition of what is required for a monoclonal antibody to be classed as a broadly neutralising antibody (bnAb) has not yet been established. This has led to hundreds of antibodies with varying neutralisation breadth being studied and has given insight into antibody maturation pathways and epitopes targeted. However, even with this knowledge, immunisation studies and vaccination trials to date have had limited success in eliciting antibodies with neutralisation breadth. For this reason there is a growing need to identify factors specifically associated with bnAb development, yet to do this a set of criteria is necessary to distinguish bnAbs from non-bnAbs. This review aims to define what it means to be a HIV-1 bnAb by comparing neutralisation breadth, genetic features and epitopes of bnAbs, and in the process highlights the challenges of comparing the array of antibodies that have been isolated over the years.
Keywords: HIV-1; broadly neutralising antibody; complementary determining region; epitope; somatic hypermutation.
Copyright © 2021 Griffith and McCoy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, et al. . Broadly Neutralizing Antibody PGT121 Allosterically Modulates CD4 Binding via Recognition of the HIV-1 Gp120 V3 Base and Multiple Surrounding Glycans. PloS Pathog (2013) 9(5):e1003342. doi: 10.1371/journal.ppat.1003342 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

