Mucosal-Associated Invariant T (MAIT) Cell Dysfunction and PD-1 Expression in Prostate Cancer: Implications for Immunotherapy
- PMID: 34737749
- PMCID: PMC8560687
- DOI: 10.3389/fimmu.2021.748741
Mucosal-Associated Invariant T (MAIT) Cell Dysfunction and PD-1 Expression in Prostate Cancer: Implications for Immunotherapy
Abstract
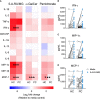
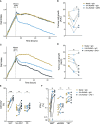
Prostate cancer is the second most common cancer in men worldwide. Despite an abundance of prostate-specific antigens, immunotherapies have yet to become a standard of care, potentially limited by T-cell dysfunction. Up to 10% of human circulating T-cells, and a significant fraction in the urogenital tract, are mucosal-associated invariant T (MAIT) cells. MAIT cells express stereotyped T-cell receptors that recognize riboflavin metabolites derived from microbes presented by MR-1. We evaluated the number, phenotype and function of circulating MAIT cells, alongside two other innate-like T (ILT) -cell subsets, in men with prostate cancer and age- and sex-matched controls. MAIT cells in men with prostate cancer circulated at similar frequencies to controls, but their cytokine production and proliferation was impaired. In contrast, the function of two other ILT-cell populations (natural killer T-cells and Vγ9Vδ2 T-cells) was not impaired. In both patients and controls, MAIT cells expressed high levels of the immune checkpoint molecule PD-1 at rest, while upregulation of PD-1 in response to the MR-1 ligand 5-amino-6D-ribitylaminouracil (5-A-RU) was greater in patients. 5-A-RU also induced upregulation of PD-L1 and -L2 RNA in primary mononuclear cells. We confirmed that circulating MAIT cell number and function were preserved before and during anti-PD1 therapy with pembrolizumab in a cohort of patients with melanoma. In vitro, 5-A-RU enhanced mononuclear cell cytotoxicity against the PD-L1 positive prostate cancer cell line PC3 in an MR-1-dependent manner. Addition of pembrolizumab enhanced this cytotoxicity, and was associated with increased MAIT cell expression of CD107a and IFN-γ. We conclude that prostate cancer is associated with MAIT-cell dysfunction, and that this might be overcome through the application of potent MR-1 ligands with PD-1 blockade. These findings may have implications for the development of cancer immunotherapies that exploit MAIT cells.
Keywords: MR1 protein; PD-1 protein; gamma delta T cells; human; mucosal-associated invariant T cells; natural killer T-cells; pembrolizumab; prostate cancer.
Copyright © 2021 Jarvis, Collings, Authier-Hall, Dasyam, Luey, Nacey, Painter, Delahunt, Hermans and Weinkove.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. . Ipilimumab Versus Placebo After Radiotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer That had Progressed After Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2014) 15:700–12. doi: 10.1016/S1470-2045(14)70189-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

