Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells
- PMID: 34737813
- PMCID: PMC8561765
- DOI: 10.3892/etm.2021.10908
Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells
Abstract
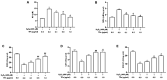
Tea polyphenols (TPs) are the major bioactive extract from green tea that have been extensively reported to prevent and treat oxidative stress damage. In previous studies, TPs have been demonstrated to protect cells against oxidative injury induced by hydrogen peroxide (H2O2). However, the underlying mechanism remains unclear. The aim of the current study was to investigate whether the protective and regulatory effects of TPs on oxidative stress damage were dependent on the mammalian STE20-like protein kinase (Mst)/nuclear factor (erythroid-derived 2)-like 2 (Nrf2) axis and the Kelch-like ECH-associated protein 1 (Keap1)/Nrf2/heme oxygenase 1 (HO-1) pathway in RAW264.7 cells, a murine macrophage cell line. Maintaining a certain range of intracellular reactive oxygen species (ROS) levels is critical to basic cellular activities, while excessive ROS generation can override the antioxidant capacity of the cell and result in oxidative stress damage. The inhibition of ROS generation offers an effective target for preventing oxidative damage. The results of the present study revealed that pretreatment with TPs inhibited the production of intracellular ROS and protected RAW264.7 cells from H2O2-induced oxidative damage. TPs was also demonstrated to attenuate the production of nitric oxide and malondialdehyde and increase the levels of antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase). In addition, following TPs treatment, alterations in Mst1/2 at the mRNA and protein level inhibited the production of ROS and promoted the self-regulation of antioxidation. TPs-induced Keap1 gene downregulation also increased the expression of Nrf2 and HO-1. Collectively, the results of the present study demonstrated that TPs provided protection against H2O2-induced oxidative injury in RAW264.7 cells.
Keywords: Kelch-like ECH-associated protein 1/nuclear factor (erythroid-derived 2)-like 2/heme oxygenase 1 axis; hydrogen peroxide; mammalian STE20-like protein kinase/nuclear factor (erythroid-derived 2)-like 2 axis; oxidative stress damage; reactive oxygen species; tea polyphenols.
Copyright: © Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Namal Senanayake SPJ. Green tea extract: Chemistry, antioxidant properties and food applications - A review. J Funct Foods. 2013;5:1529–1541.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
