Immunofluorescence-guided segmentation of three-dimensional features in micro-computed tomography datasets of human lung tissue
- PMID: 34737879
- PMCID: PMC8564621
- DOI: 10.1098/rsos.211067
Immunofluorescence-guided segmentation of three-dimensional features in micro-computed tomography datasets of human lung tissue
Abstract
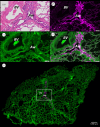
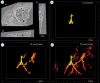
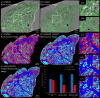
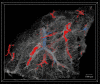
Micro-computed tomography (µCT) provides non-destructive three-dimensional (3D) imaging of soft tissue microstructures. Specific features in µCT images can be identified using correlated two-dimensional (2D) histology images allowing manual segmentation. However, this is very time-consuming and requires specialist knowledge of the tissue and imaging modalities involved. Using a custom-designed µCT system optimized for imaging unstained formalin-fixed paraffin-embedded soft tissues, we imaged human lung tissue at isotropic voxel sizes less than 10 µm. Tissue sections were stained with haematoxylin and eosin or cytokeratin 18 in columnar airway epithelial cells using immunofluorescence (IF), as an exemplar of this workflow. Novel utilization of tissue autofluorescence allowed automatic alignment of 2D microscopy images to the 3D µCT data using scripted co-registration and automated image warping algorithms. Warped IF images, which were accurately aligned with the µCT datasets, allowed 3D segmentation of immunoreactive tissue microstructures in the human lung. Blood vessels were segmented semi-automatically using the co-registered µCT datasets. Correlating 2D IF and 3D µCT data enables accurate identification, localization and segmentation of features in fixed soft lung tissue. Our novel correlative imaging workflow provides faster and more automated 3D segmentation of µCT datasets. This is applicable to the huge range of formalin-fixed paraffin-embedded tissues held in biobanks and archives.
Keywords: SIFT; blood vessel networks; correlative imaging; histology; registration; warping.
© 2021 The Authors.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
