Rho GTPase signalling networks in cancer cell transendothelial migration
- PMID: 34738075
- PMCID: PMC8558887
- DOI: 10.1530/VB-21-0008
Rho GTPase signalling networks in cancer cell transendothelial migration
Abstract
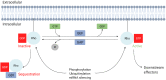
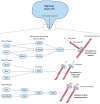
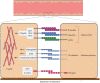
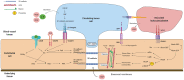
Rho GTPases are small signalling G-proteins that are central regulators of cytoskeleton dynamics, and thereby regulate many cellular processes, including the shape, adhesion and migration of cells. As such, Rho GTPases are also essential for the invasive behaviour of cancer cells, and thus involved in several steps of the metastatic cascade, including the extravasation of cancer cells. Extravasation, the process by which cancer cells leave the circulation by transmigrating through the endothelium that lines capillary walls, is an essential step for metastasis towards distant organs. During extravasation, Rho GTPase signalling networks not only regulate the transmigration of cancer cells but also regulate the interactions between cancer and endothelial cells and are involved in the disruption of the endothelial barrier function, ultimately allowing cancer cells to extravasate into the underlying tissue and potentially form metastases. Thus, targeting Rho GTPase signalling networks in cancer may be an effective approach to inhibit extravasation and metastasis. In this review, the complex process of cancer cell extravasation will be discussed in detail. Additionally, the roles and regulation of Rho GTPase signalling networks during cancer cell extravasation will be discussed, both from a cancer cell and endothelial cell point of view.
Keywords: Rho GTPase; cancer; endothelium; extravasation; invadopodia; metastasis.
© The authors.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

