Risk evaluation of denosumab and zoledronic acid for medication-related osteonecrosis of the jaw in patients with bone metastases: a propensity score-matched analysis
- PMID: 34738163
- PMCID: PMC8794983
- DOI: 10.1007/s00520-021-06634-7
Risk evaluation of denosumab and zoledronic acid for medication-related osteonecrosis of the jaw in patients with bone metastases: a propensity score-matched analysis
Abstract
Purpose: This study evaluated the risk of medication-related osteonecrosis of the jaw (MRONJ) in patients with cancer who received denosumab or zoledronic acid (ZA) for treating bone metastasis.
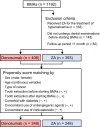
Methods: The medical records of patients were retrospectively reviewed. Patients who did not undergo a dental examination at baseline were excluded. The primary endpoint was a comparison of the risk of developing MRONJ between the denosumab and ZA groups. Propensity score matching was used to control for baseline differences between patient characteristics and compare outcomes for both groups.
Results: Among the 799 patients enrolled, 58 (7.3%) developed MRONJ. The incidence of MRONJ was significantly higher in the denosumab group than in the ZA group (9.6% [39/406] vs. 4.8% [19/393], p = 0.009). Multivariate Cox proportional hazards regression analysis revealed that denosumab treatment (hazard ratio [HR], 2.89; 95% confidence interval [CI], 1.65-5.25; p < 0.001) and tooth extraction after starting ZA or denosumab (HR, 4.26; 95% CI, 2.38-7.44; p < 0.001) were significant risk factors for MRONJ. Propensity score-matched analysis confirmed that the risk of developing MRONJ was significantly higher in the denosumab group than in the ZA group (HR, 2.34; 95% CI, 1.17-5.01; p = 0.016).
Conclusion: The results of this study suggest that denosumab poses a significant risk for developing MRONJ in patients treated for bone metastasis, and thus these patients require close monitoring.
Keywords: Denosumab; Osteonecrosis of the jaw; Risk factor; Zoledronic acid.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Saito G, Ebata T, Ishiwata T, Iwasawa S, Yoshino I, Takiguchi Y, Tatsumi K. Risk factors for skeletal-related events in non-small cell lung cancer patients treated with bone-modifying agents. Support Care Cancer. 2021;29:4081–4088. - PubMed
-
- von Moos R, Costa L, Gonzalez-Suarez E, Terpos E, Niepel D, Body JJ. Management of bone health in solid tumours: from bisphosphonates to a monoclonal antibody. Cancer Treat Rev. 2019;76:57–67. - PubMed
-
- Link H, Diel I, Ohlmann CH, Holtmann L, Kerkmann M; Associations Supportive Care in Oncology (AGSMO), Medical Oncology (AIO), Urological Oncology (AUO), within the German Cancer Society (DKG) and the German Osteooncological Society (DOG) (2020) Guideline adherence in bone-targeted treatment of cancer patients with bone metastases in Germany. Support Care Cancer 28:2175–2184 - PMC - PubMed
-
- Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, ESMO Guidelines Working Group (2014) Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol 25: iii124–137 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

